GENTLE RAIN ANTIBACTERIAL - triclosan shampoo
Coloplast Manufacturing US, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
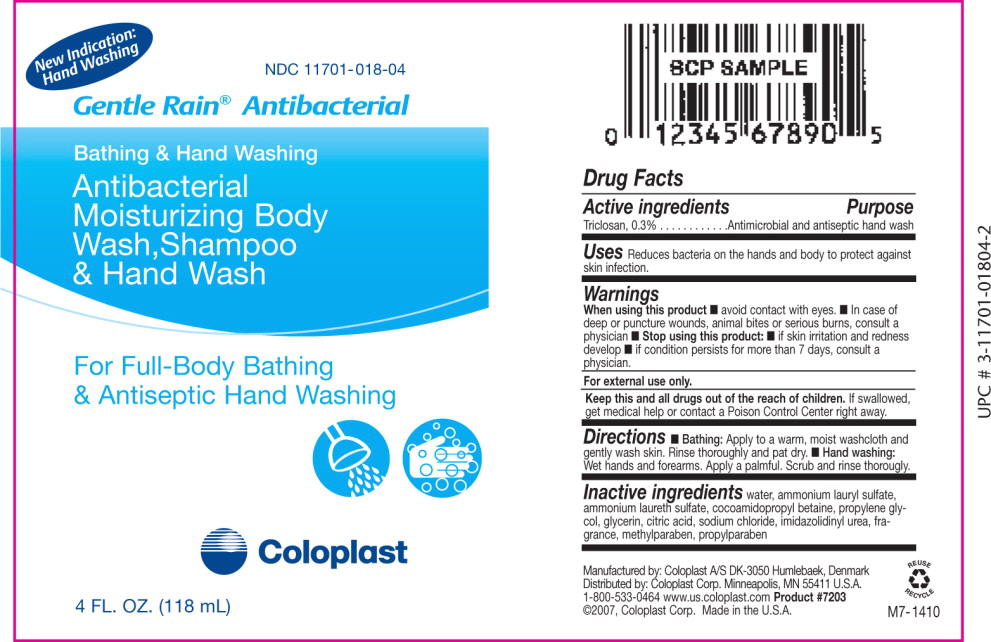
Gentle Rain®Antibacterial
New Indication:
Hand Washing
Bathing & Hand Washing
Antibacterial Moisturizing Body Wash, Shampoo & Hand Wash
Drug Facts
When using this product
- avoid contact with eyes.
- In case of deep or puncture wounds, animal bites or serious burns, consult a physician
Stop using this product:
- if skin irritation and redness develop
- if condition persists for more than 7 days, consult a physician.
Keep this and all drugs out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Bathing: Apply to a warm, moist washcloth and gently wash skin. Rinse thoroughly and pat dry.
- Hand washing: Wet hands and forearms. Apply a palmful. Scrub and rinse thoroughly.
Inactive ingredients water, ammonium lauryl sulfate ammonium laureth sulfate, cocoamidopropyl betaine, propylene glycol, glycerin, citric acid, sodium chloride, imidazolidinyl urea fragrance, methylparaben, propylparaben
Manufactured by: Coloplast A/S DK-3050 Humlebaek, Denmark
Distributed by: Coloplast Corp. Minneapolis, MN 55411 U.S.A.
1-800-533-0464 www.us.coloplast.com
Product #7201 ©2007, Coloplast Corp. Made in the U.S.A.
H7-996
| GENTLE RAIN ANTIBACTERIAL
triclosan shampoo |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Coloplast Manufacturing US, LLC (110326675) |
| Registrant - Coloplast Corp (847436391) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Coloplast Manufacturing US, LLC | 110326675 | MANUFACTURE(11701-018) | |