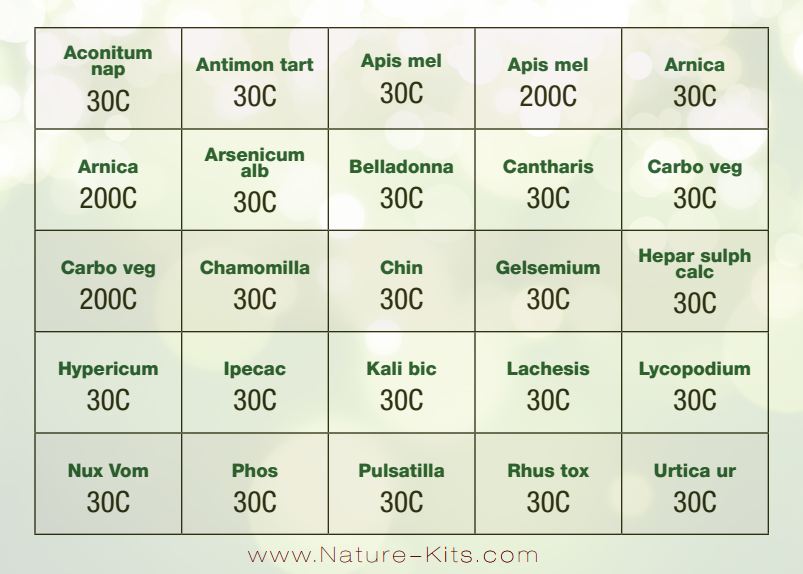
Label: ACUTE CARE KIT- aconitum napellus, antimonium tartaricum, apis mellifica, arnica montana, arsenicum album, belladonna, cantharis, carbo vegetabilis, chamomilla, cinchona, gelsemium, hepar sulph calcareum, hypericum perforaturm, ipecacuanha, kali bichromicum, lachesis mutus, lycopodium clavatum, nux vomica, phosphorus, pulsatilla, rhus toxicodendron, urtica urens pellet
-
Contains inactivated NDC Code(s)
NDC Code(s): 62990-690-18 - Packager: MARPE LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 30, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS (HPUS*): ACONITUM NAPELLUS 30C, ANTIMONIUM TARTARICUM 30C, APIS MELLIFICA 30C, APIS MELLIFICA 200C, ARNICA MONTANA 30C, ARNICA MONTANA 200C, ARSENICUM ALBUM 30C, BELLADONNA 30C, CANTHARIS 30C, CARBO VEGETABILIS 30C, CARBO VEGETABILIS 200C, CHAMOMILLA 30C, CHINA OFFICINALIS 30C, GELSEMIUM SEMPERVIRENS 30C, HEPAR SULPHURIS CALCAREUM 30C, HYPERICUM PERFORATUM 30C, IPECACUANHA 30C, KALI BICHROMICUM 30C, LACHESIS MUTUS 30C, LYCOPODIUM CLAVATUM 30C, NUX VOMICA 30C, PHOSPHORUS 30C, PULSATILLA 30C, RHUS TOXICODENDRON 30C, URTICA URENS 30C.
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- WARNINGS
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACUTE CARE KIT
aconitum napellus, antimonium tartaricum, apis mellifica, arnica montana, arsenicum album, belladonna, cantharis, carbo vegetabilis, chamomilla, cinchona, gelsemium, hepar sulph calcareum, hypericum perforaturm, ipecacuanha, kali bichromicum, lachesis mutus, lycopodium clavatum, nux vomica, phosphorus, pulsatilla, rhus toxicodendron, urtica urens pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62990-690 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 30 [hp_C] ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 30 [hp_C] ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] LYTTA VESICATORIA (UNII: 3Q034RO3BT) (LYTTA VESICATORIA - UNII:3Q034RO3BT) LYTTA VESICATORIA 30 [hp_C] ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 30 [hp_C] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 30 [hp_C] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_C] POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 30 [hp_C] URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62990-690-18 25 in 1 KIT Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/21/2014 Labeler - MARPE LLC (006609105) Registrant - OHM PHARMA INC. (030572478) Establishment Name Address ID/FEI Business Operations OHM PHARMA INC. 030572478 manufacture(62990-690)