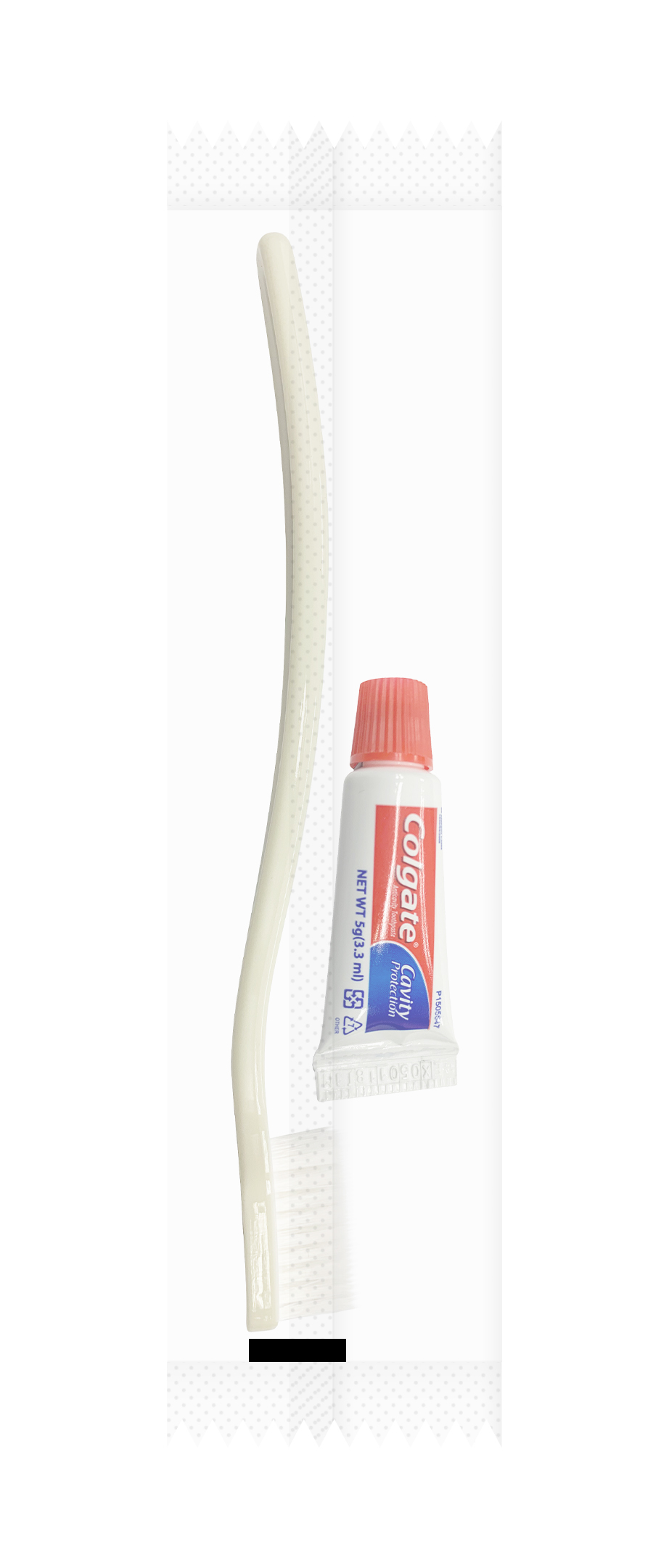
Label: 5G COLGATE PLUS TOOTHBRUSH KIT- sodium monofluorophosphate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42555-000-01, 42555-060-94 - Packager: Colgate-Palmolive (Thailand) Limited - Branch
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
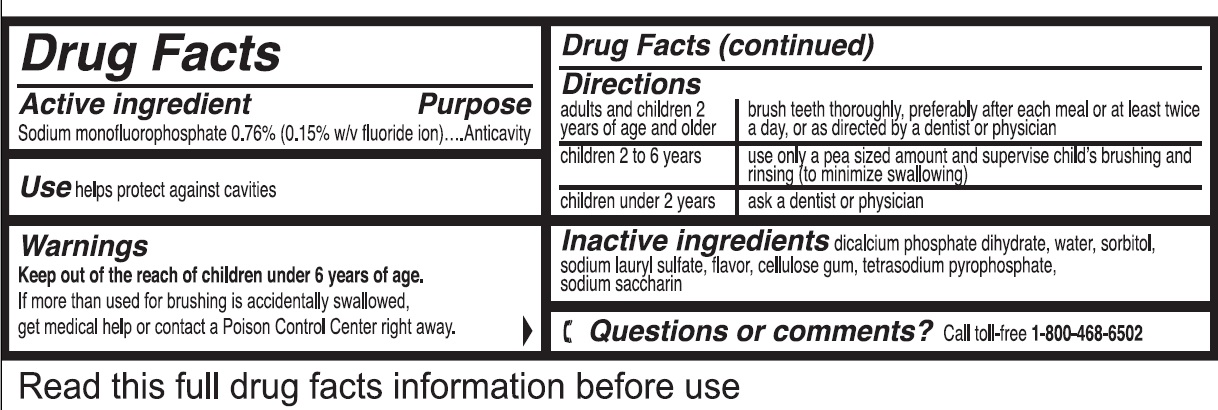
- Drug Facts
- Active ingredient
- Use
- Warnings
-
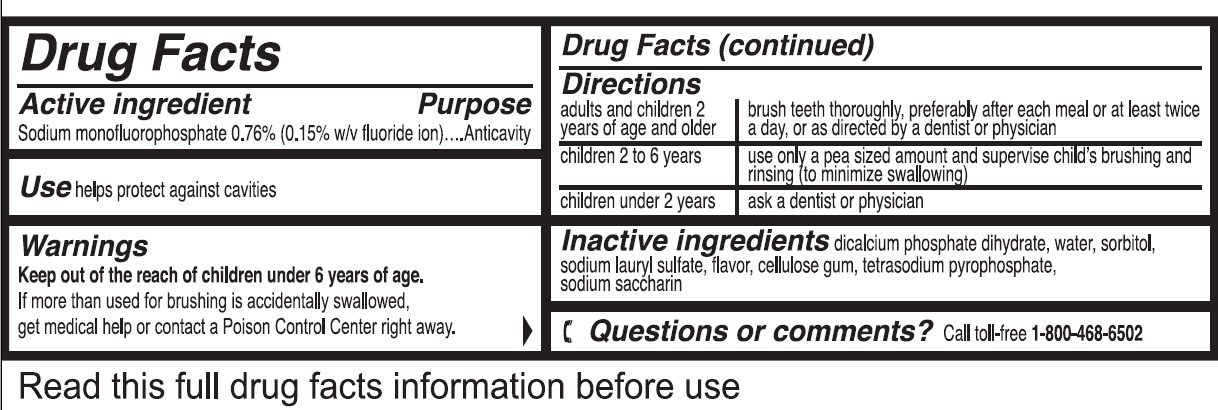
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
5G COLGATE PLUS TOOTHBRUSH KIT
sodium monofluorophosphate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42555-000 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42555-000-01 1 in 1 KIT 06/07/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 5 g Part 1 of 1 COLGATE ANTICAVITY
sodium monofluorophosphate paste, dentifriceProduct Information Item Code (Source) NDC:42555-060 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 7.6 mg in 1 g Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) METHYL SALICYLATE (UNII: LAV5U5022Y) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color white Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42555-060-94 5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 06/07/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 06/07/2018 Labeler - Colgate-Palmolive (Thailand) Limited - Branch (672044552) Establishment Name Address ID/FEI Business Operations Luoding Quality Amenities Supply Limited 528126807 manufacture(42555-000)