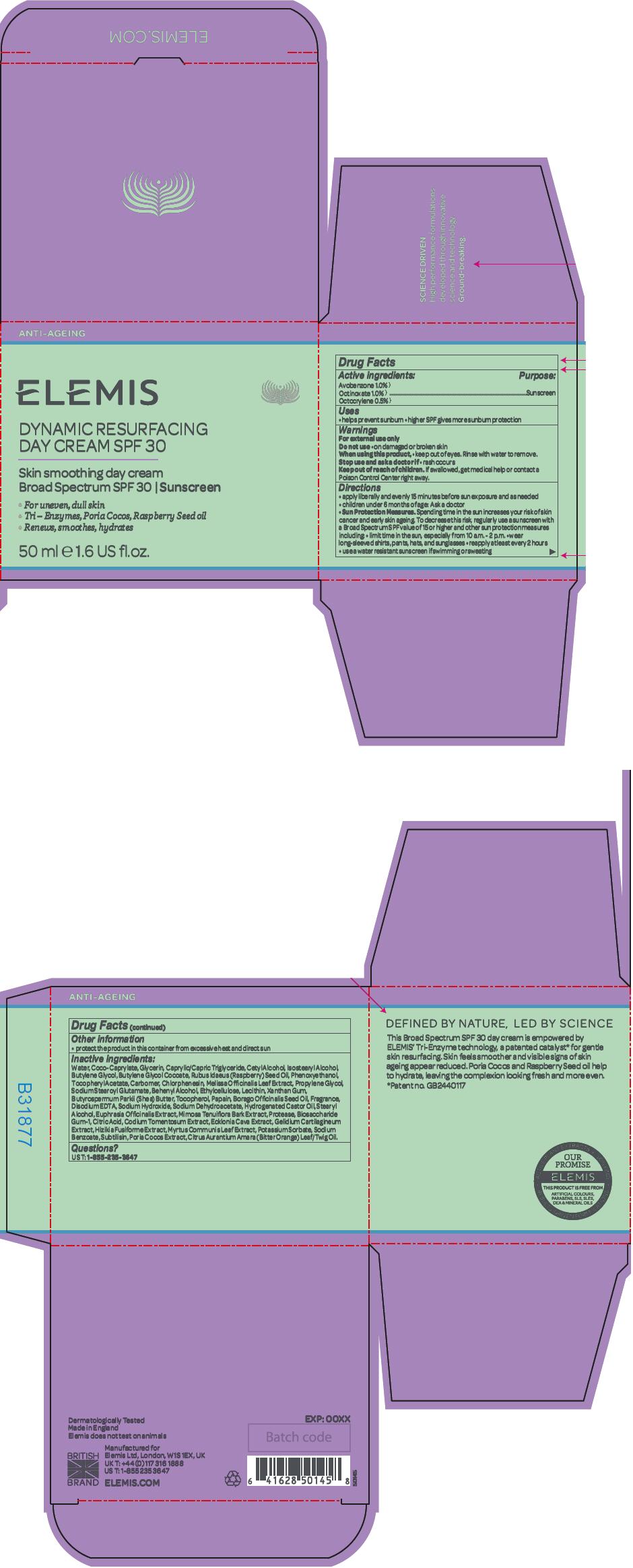
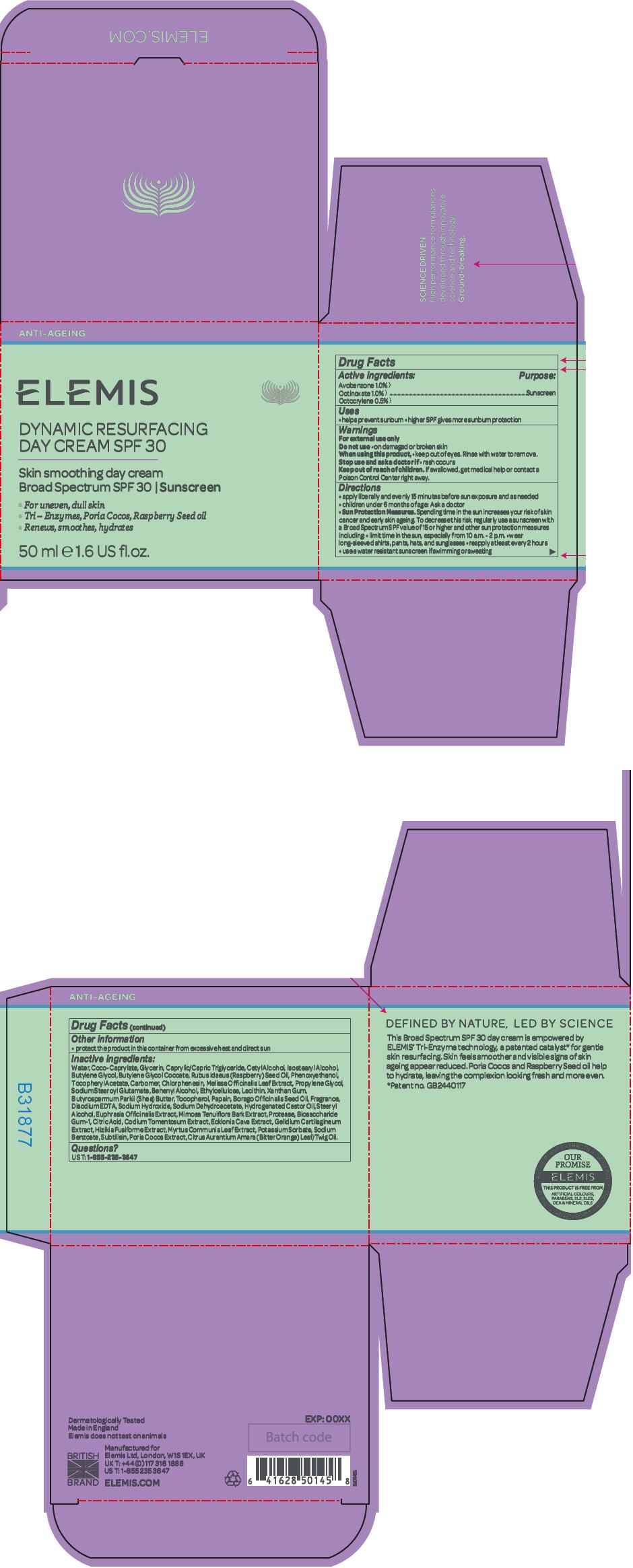
Label: DYNAMIC RESURFACING SPF 30- avobenzone, octinoxate, and octocrylene cream
- NDC Code(s): 65912-002-50, 65912-002-51, 65912-002-52
- Packager: ELEMIS Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally and evenly 15 minutes before sun exposure and as needed
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Other information
-
Inactive ingredients
Water, Coco-Caprylate, Glycerin, Caprylic/Capric Triglyceride, Cetyl Alcohol, Isostearyl Alcohol, Butylene Glycol, Butylene Glycol Cocoate, Rubus Idaeus (Raspberry) Seed Oil, Phenoxyethanol, Tocopheryl Acetate, Carbomer, Chlorphenesin, Melissa Officinalis Leaf Extract, Propylene Glycol, Sodium Stearoyl Glutamate, Behenyl Alcohol, Ethylcellulose, Lecithin, Xanthan Gum, Butyrospermum Parkii (Shea) Butter, Tocopherol, Papain, Borago Officinalis Seed Oil, Fragrance, Disodium EDTA, Sodium Hydroxide, Sodium Dehydroacetate, Hydrogenated Castor Oil, Stearyl Alcohol, Euphrasia Officinalis Extract, Mimosa Tenuiflora Bark Extract, Protease, Biosaccharide Gum-1, Citric Acid, Codium Tomentosum Extract, Ecklonia Cava Extract, Gelidium Cartilagineum Extract, Hizikia Fusiforme Extract, Myrtus Communis Leaf Extract, Potassium Sorbate, Sodium Benzoate, Subtilisin, Poria Cocos Extract, Citrus Aurantium Amara (Bitter Orange) Leaf/Twig Oil.
- Questions?
- PRINCIPAL DISPLAY PANEL - 50 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
DYNAMIC RESURFACING SPF 30
avobenzone, octinoxate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65912-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 10 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 10 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE (UNII: 4828G836N6) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETYL ALCOHOL (UNII: 936JST6JCN) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) RASPBERRY SEED OIL (UNII: 9S8867952A) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CHLORPHENESIN (UNII: I670DAL4SZ) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) DOCOSANOL (UNII: 9G1OE216XY) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) XANTHAN GUM (UNII: TTV12P4NEE) SHEA BUTTER (UNII: K49155WL9Y) TOCOPHEROL (UNII: R0ZB2556P8) PAPAIN (UNII: A236A06Y32) BORAGE SEED OIL (UNII: F8XAG1755S) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) EUPHRASIA STRICTA (UNII: C9642I91WL) MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CODIUM TOMENTOSUM (UNII: B8B45BRU87) ECKLONIA CAVA (UNII: UXX2N5V39P) GELIDIUM CARTILAGINEUM (UNII: 37LBZ0E1UE) SARGASSUM FUSIFORME (UNII: X436TJ4A11) MYRTUS COMMUNIS LEAF (UNII: U20N87188F) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) SUBTILISIN (UNII: 0ZP13EBC5Y) FU LING (UNII: XH37TWY5O4) CITRUS AURANTIUM LEAFY TWIG OIL (UNII: 5K6H1IMT3D) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65912-002-50 1 in 1 CARTON 03/05/2018 1 50 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:65912-002-51 50 g in 1 JAR; Type 0: Not a Combination Product 03/05/2018 3 NDC:65912-002-52 50 g in 1 JAR; Type 0: Not a Combination Product 03/05/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/05/2018 Labeler - ELEMIS Limited (399838895)