TRIPLE ANTIBIOTIC- bacitracin zinc and neomycin sulfate and polymyxin b sulfate ointment
Preferred Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
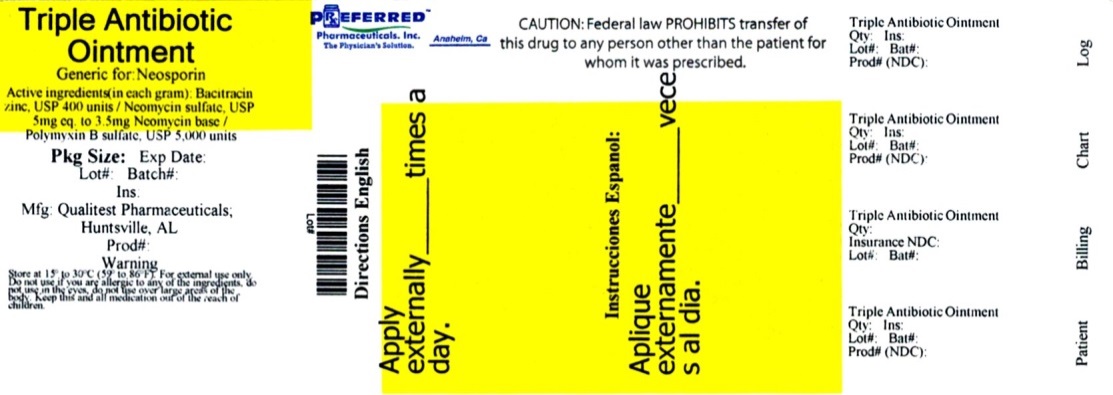
Triple Antibiotic Ointment
Active ingredients (in each gram)
Bacitracin zinc, USP 400 units
Neomycin sulfate, USP 5 mg (equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate, USP 5,000 units
Do not use
- •
- if you are allergic to any of the ingredients
- •
- in the eyes
- •
- over large areas of the body
Stop use and ask a doctor if
- •
- you need to use longer than 1 week
- •
- condition persists or gets worse
- •
- rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- clean the affected area
- •
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- •
- may be covered with a sterile bandage
Other information
- •
- store at 15° to 30°C (59° to 86°F)
- •
- lot number and expiration date: See crimp of tube or box
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
| TRIPLE ANTIBIOTIC
bacitracin zinc and neomycin sulfate and polymyxin b sulfate ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-8995) | |
Revised: 11/2017
Document Id: 6989ccf8-6cb0-4d1a-8cd3-f3b8fd3a50ad
Set id: fbaa35f6-6ef6-4a9b-81c7-03f1a0151c32
Version: 2
Effective Time: 20171116
Preferred Pharmaceuticals Inc.