MEDICATED SOFT N SURE HEALTHCARE PERSONNEL HANDWASH- triclosan liquid
Deb USA, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
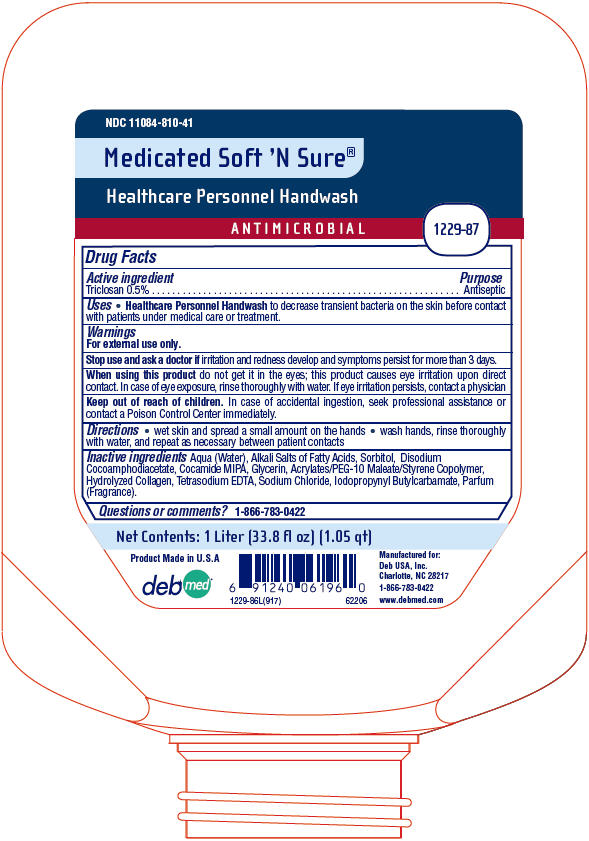
Medicated Soft 'N Sure®
Healthcare Personnel Handwash
Uses
- Healthcare Personnel Handwash to decrease transient bacteria on the skin before contact with patients under medical care or treatment.
Warnings
For external use only.
Stop use and ask a doctor if irritation and redness develop and symptoms persist for more than 3 days.
Directions
- wet skin and spread a small amount on the hands
- wash hands, rinse thoroughly with water, and repeat as necessary between patient contacts
| MEDICATED SOFT N SURE
HEALTHCARE PERSONNEL HANDWASH
triclosan liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Deb USA, Inc. (607378015) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| STERIS Corporation | 139424188 | MANUFACTURE(11084-810) | |
Revised: 9/2019
Document Id: 1c298787-a1c3-47d2-8bdd-e0066702a4f8
Set id: fb6db121-b1aa-46c6-9092-e5e887b92d7b
Version: 2
Effective Time: 20190927
Deb USA, Inc.