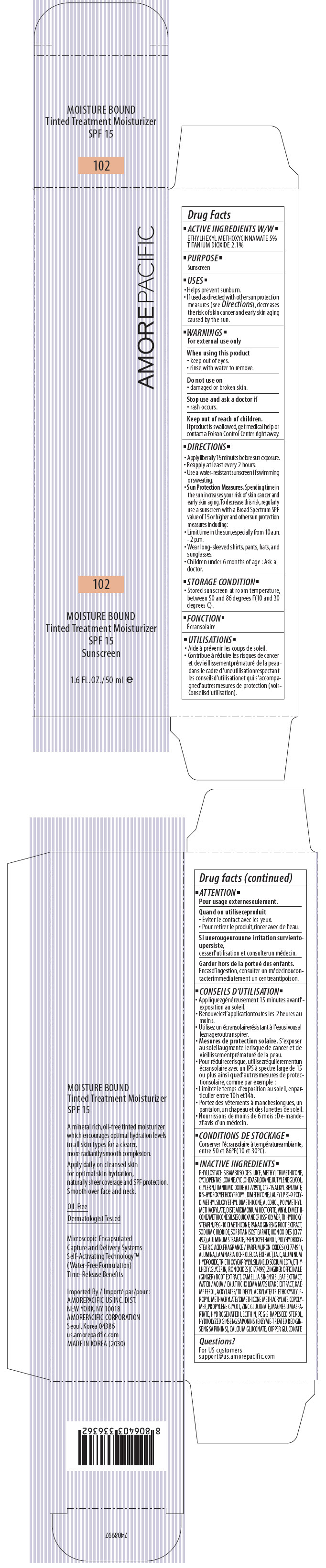
AMOREPACIFIC MOISTURE BOUND TINTED TREATMENT MOISTURIZER NO. 102- octinoxate and titanium dioxide lotion
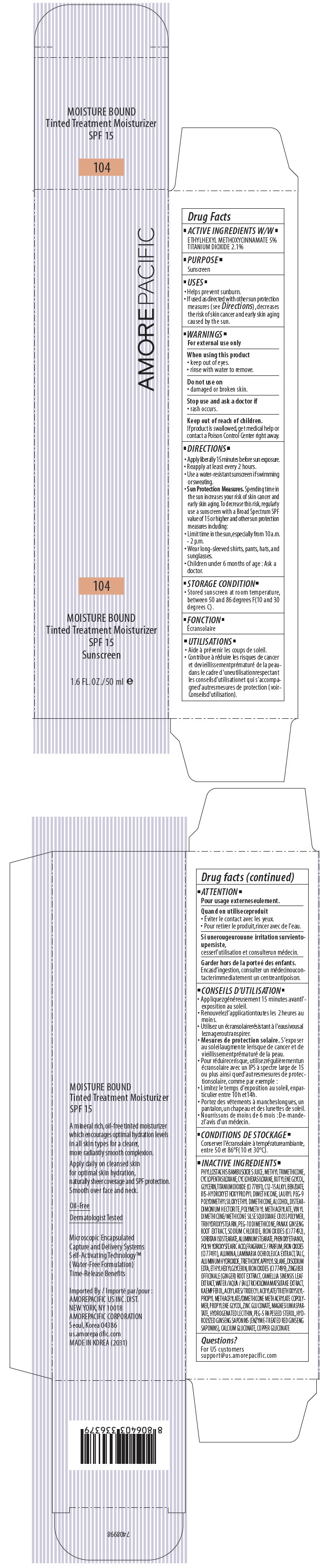
AMOREPACIFIC MOISTURE BOUND TINTED TREATMENT MOISTURIZER NO. 104- octinoxate and titanium dioxide lotion
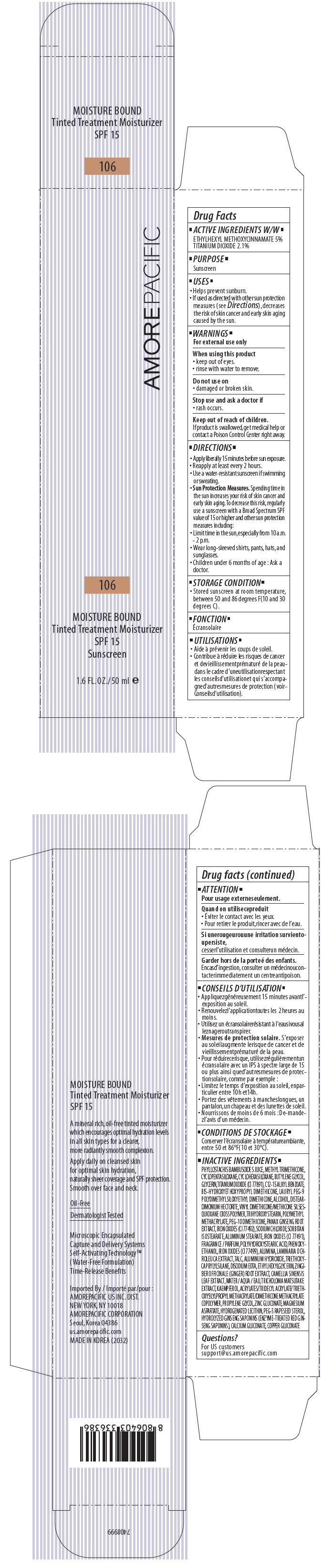
AMOREPACIFIC MOISTURE BOUND TINTED TREATMENT MOISTURIZER NO. 106- octinoxate and titanium dioxide lotion
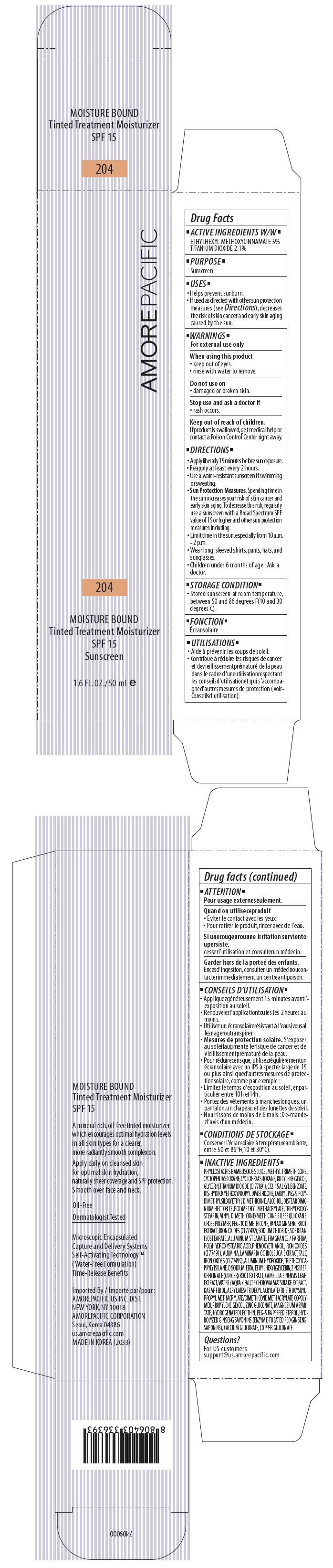
AMOREPACIFIC MOISTURE BOUND TINTED TREATMENT MOISTURIZER NO. 204- octinoxate and titanium dioxide lotion
AMOREPACIFIC Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENTS W/W
ETHYLHEXYL METHOXYCINNAMATE 5%
TITANIUM DIOXIDE 2.1%
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
WARNINGS
For external use only
When using this product
- keep out of eyes.
- rinse with water to remove.
Stop use and ask a doctor if
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age : Ask a doctor.
STORAGE CONDITION
- Stored sunscreen at room temperature, between 50 and 86 degrees F(10 and 30 degrees C).
INACTIVE INGREDIENTS
PHYLLOSTACHIS BAMBUSOIDES JUICE, METHYL TRIMETHICONE, CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, BUTYLENE GLYCOL, GLYCERIN, TITANIUM DIOXIDE (CI 77891), C12-15 ALKYL BENZOATE, BIS-HYDROXYETHOXYPROPYL DIMETHICONE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, ALCOHOL, POLYMETHYL METHACRYLATE, DISTEARDIMONIUM HECTORITE, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, TRIHYDROXYSTEARIN, PEG-10 DIMETHICONE, PANAX GINSENG ROOT EXTRACT, SODIUM CHLORIDE, SORBITAN ISOSTEARATE, IRON OXIDES (CI 77 492), ALUMINUM STEARATE, PHENOXYETHANOL, POLYHYDROXYSTEARIC ACID, FRAGRANCE / PARFUM, IRON OXIDES (CI 77491), ALUMINA, LAMINARIA OCHROLEUCA EXTRACT, TALC, ALUMINUM HYDROXIDE, TRIETHOXYCAPRYLYLSILANE, DISODIUM EDTA, ETHYLHEXYLGLYCERIN, IRON OXIDES (CI 77499), ZINGIBER OFFICINALE (GINGER) ROOT EXTRACT, CAMELLIA SINENSIS LEAF EXTRACT, WATER / AQUA / EAU, TRICHOLOMA MATSUTAKE EXTRACT, KAEMPFEROL, ACRYLATES/TRIDECYL ACRYLATE/TRIETHOXYSILYLPROPYL METHACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, PROPYLENE GLYCOL, ZINC GLUCONATE, MAGNESIUM ASPARTATE, HYDROGENATED LECITHIN, PEG-5 RAPESEED STEROL, HYDROLYZED GINSENG SAPONINS (ENZYME-TREATED RED GINSENG SAPONINS), CALCIUM GLUCONATE, COPPER GLUCONATE
Questions?
For US customers support@us.amorepacific.com
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - 102
AMOREPACIFIC
102
MOISTURE BOUND
Tinted Treatment Moisturizer
SPF 15
Sunscreen
1.6 FL. OZ./ 50 ml e
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - 104
AMOREPACIFIC
104
MOISTURE BOUND
Tinted Treatment Moisturizer
SPF 15
Sunscreen
1.6 FL. OZ./ 50 ml e
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - 106
AMOREPACIFIC
106
MOISTURE BOUND
Tinted Treatment Moisturizer
SPF 15
Sunscreen
1.6 FL. OZ./ 50 ml e
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - 204
AMOREPACIFIC
204
MOISTURE BOUND
Tinted Treatment Moisturizer
SPF 15
Sunscreen
1.6 FL. OZ./ 50 ml e