Label: DRAX EXAMETAZIME- kit for the preparation of technetium tc 99m exametazime for leukocyte labeling injection, powder, lyophilized, for solution
- NDC Code(s): 65174-200-01, 65174-200-05
- Packager: Jubilant DraxImage Inc., dba Jubilant Radiopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Drax Exametazime safely and effectively. See full prescribing information for Drax Exametazime.
Drax Exametazime (kit for the preparation of technetium Tc 99m exametazime for leukocyte labeling) for intravenous use.
Initial U.S. Approval: 1988INDICATIONS AND USAGE
Drax Exametazime is a radioactive diagnostic agent indicated for leukocyte (white blood cell) labeled scintigraphy as an adjunct in the localization of intra-abdominal infection and inflammatory bowel disease. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Five (5) single-dose vials, each vial contains: 370 MBq up to 2000 MBq (10 mCi up to 54 mCi) [74 - 370 MBq / mL (2 - 10 mCi / mL)] at time of preparation (reconstitution). (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Hypersensitivity reactions, including serious signs and symptoms of anaphylaxis, following administration of Tc 99m labeled leukocytes prepared using Tc 99m exametazime have been reported. Always have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions. (5.1)
ADVERSE REACTIONS
Most common adverse reactions include transient increase in blood pressure, rash, generalized erythema, facial edema and fever. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Jubilant DraxImage Inc. at 1-888-633-5343 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
- Lactation: Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for 12 to 24 hours after Technetium Tc 99m Exametazime labeled leukocyte administration. (8.2)
- Renal impairment: Increased radiation exposure in patients with impaired renal function. A reduction in administered Tc 99m can be considered provided adequate numbers of labeled WBCs are administered (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Important Administration Instructions
2.3 Recommended Dosage and Administration
2.4 Image Acquisition and Inerpretation
2.5 Preparation of Autologous Leukocytes
2.6 Labeling of Autologous Leukocytes with Technetium Tc 99m Exametazime
2.7 Preparation of Technetium Tc 99m Exametazime
2.8 Radiochemical Purity Testing - Quality control of Tc 99m Exametazime
2.9 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Risk for Image Interpretation Error
5.3 Radiation Exposure Risk
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
Technetium Tc 99m exametazime is a radioactive solution and should be handled with appropriate safety measures to minimize radiation exposure. During handling use waterproof gloves and effective shielding, including syringe shields [see Warnings and Precautions (5.3)].
2.2 Important Administration Instructions
- Use strict aseptic procedures throughout preparation and handling.
- Visually inspect the reconstituted technetium Tc 99m exametazime solution for particulate matter and discoloration prior to radiolabeling of white blood cells. Do not use the reconstituted solution if there is evidence of particulate matter or discoloration.
- Follow the directions of drug preparation carefully to ensure efficient leukocytes labeling [see Dosage and Administration (2.5, 2.6)].
- Measure patient dose with a suitable radioactivity calibration system immediately prior to administration.
- Instruct patients to hydrate, after administration of technetium Tc 99m exametazime labeled white blood cells and void frequently to minimize radiation dose to the kidneys and bladder [see Warnings and Precautions (5.3)].
2.3 Recommended Dosage and Administration
For an adult patient the recommended intravenous injection dose range for technetium Tc 99m exametazime labeled leukocytes is 259 - 925 Megabecquerels (MBq) [7-25 millicuries (mCi)].
2.4 Image Acquisition and Inerpretation
Acquisition
- Instruct patients to empty their bladder prior to imaging.
- Obtain serial pelvic and abdominal images beginning at 0.5 – 1 hour post-injection and continue up to 4 hours.
Interpretation
- Accumulation of radioactivity in bowel seen in early images [less than 4 hours] with increasing intensity and/or no evidence of changing location secondary to GI motility likely represents inflammatory bowel disease or infection. Radioactivity from hepatic excretion detected in the bowel 4 hours post-injection and changing in GI location on serial/subsequent images is indicative of normal GI transit [see Clinical Pharmacology (12.2)]
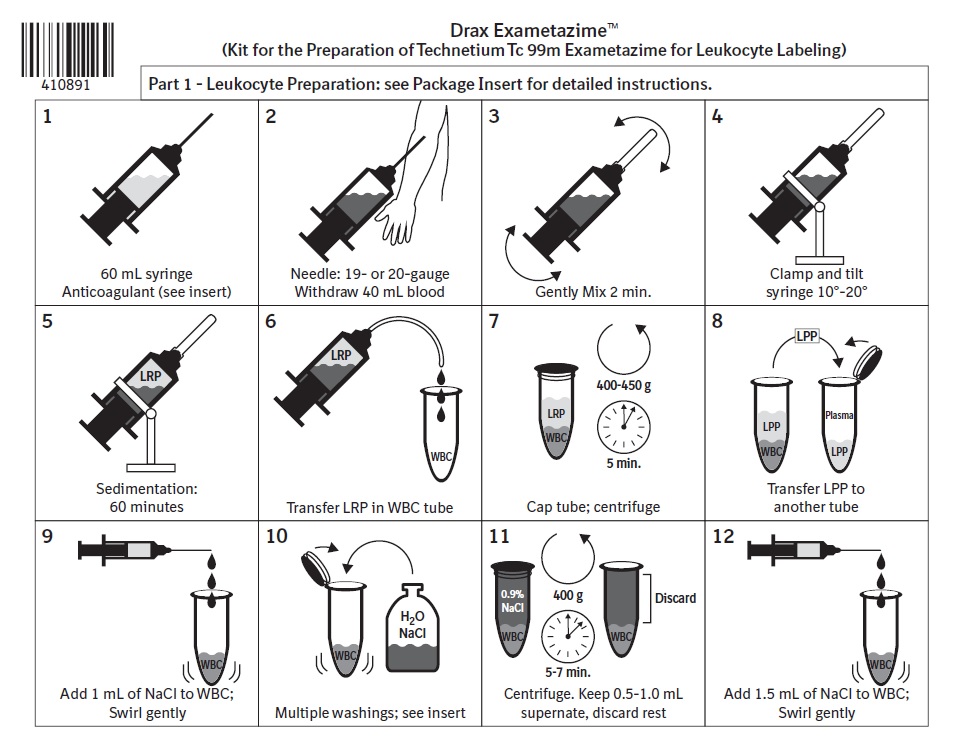
2.5 Preparation of Autologous Leukocytes
IMPORTANT - Label all syringes and tubes used in this labeling procedure with the patient’s name and unique identification number.
Leukocyte Harvest and Separation
1. Draw 2 mL of Heparin and 8 mL of 6% Hydroxyethyl starch into a 60 mL plastic syringe.
2. Withdraw approximately 40 mL whole blood from the patient into the syringe using a 19-gauge Butterfly needle infusion set. Close the syringe with a sterile hub.
3. Gently mix the contents for 2 minutes.
4. Clamp the syringe barrel to the ring stand in an upright (hub side up) position and tilt the syringe approximately 10-20 degrees from its position perpendicular to the bench.
5. Allow the syringe to stand a minimum of 60 minutes until the red blood cells sediment and the supernatant looks clear.
6. Using an infusion set, transfer the leukocyte-rich plasma (LRP), the supernatant, from the previous step, into a sterile, conical centrifuge tube marked "WBC" (white blood cell) and assure that only a minimum amount of red cells enter the centrifuge tube.
7. Immediately centrifuge the capped WBC tube at 400-450 g for 5 minutes. The plasma will separate out into a liquid [leukocyte poor plasma (LPP)] and a solid (WBC button). The WBC button often contains a small number of red blood cells and may appear red.
8. Transfer the leukocyte poor plasma (LPP) into another sterile centrifuge tube marked as "Plasma" tube, without disturbing the WBC button. Save the LPP in the Plasma tube for later use (Steps 16 and 19).Red Blood Cell Lysis and Washing
9. Add 1 mL Sodium Chloride (Na Cl) Injection, USP (0.9%) to the WBC button and suspend.
10. Add the following to the WBC suspension in succession and swirl the centrifuge tube (WBC tube) for 5-30 seconds after each addition. (Attention to timing is important as exposing leukocytes to a hypotonic solution for a prolonged period will damage leukocytes and result in poor leukocyte labeling results):
a) 9 mL sterile water;
b) 2 mL of 5% Na Cl; and
c) 10 mL of 0.9% Na Cl.
11. Cap the WBC tube and centrifuge at 400 g for 5-7 minutes. Draw off the supernatant into the "Waste" tube.
12. Add 1.5 mL of 0.9% Na Cl and re-suspend the WBC button by gentle shaking.
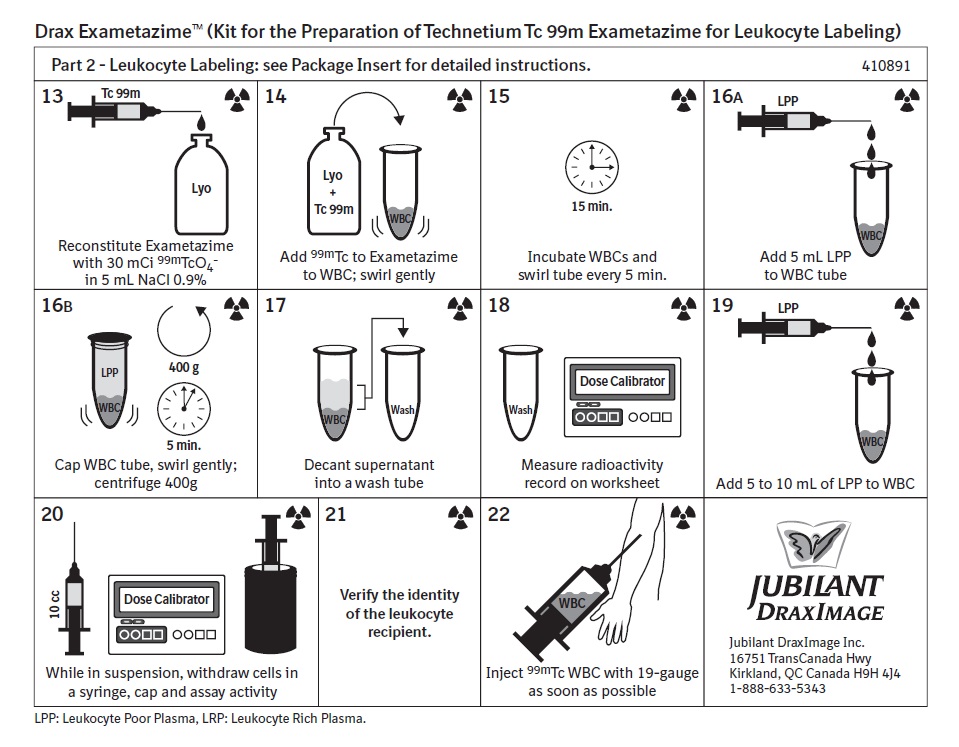
13. Reconstitute technetium Tc 99m exametazime with generator eluate [see Dosage and Administration (2.7)]. Measure the radioactivity and record as item (1) on the Technetium Tc 99m Exametazime Labeling Efficiency Worksheet. Use for radiolabeling WBC within 30 minutes.2.6 Labeling of Autologous Leukocytes with Technetium Tc 99m Exametazime
14. Carefully add the reconstituted technetium Tc 99m exametazime to the WBC tube containing the WBC button isolated in Step 12.
15. Incubate the WBCs at room temperature for 15 minutes. Swirl during the incubation every 5 minutes.
16. Add 5 mL of LPP (from Step 8) to the WBC tube. Cap the WBC tube and centrifuge at 400 g for 5 minutes.
17. Carefully remove the supernatant and place into the tube labeled “Wash.” Keep the labeled white cells in the WBC tube.
18. Measure the radioactivity of the Wash tube and record as item (2) on the Technetium Tc 99m Exametazime Labeling Efficiency Worksheet.
19. Add 5-10 mL of LPP (from Step 8) to the Tc 99m labeled leukocyte preparation (WBC tube). Gently swirl to mix.
20. Draw up the labeled cells into a non-heparinized syringe with a large bore needle (no smaller than 19-gauge) and cap it with a sterile hub. Measure the radioactivity of the cells and record as item (3) on the Technetium Tc 99m Exametazime Labeling Efficiency Worksheet.
21. Verify the identity of the leukocyte recipient.
22. Labeled cells are now ready for administration. Administer as soon as possible and preferably within 1-2 hours after labeling.
23. Calculate the labeling efficiency from the Labeling Efficiency Worksheet:Radioactivity of the cells [item (3)]
Radioactivity of the cells [item (3)] + activity in the supernatant [item (2)]Labeling efficiency >50% is anticipated.
2.7 Preparation of Technetium Tc 99m Exametazime
The technetium Tc 99m labeling reaction involved in preparing the agent depends on maintaining the stannous ion in the reduced state. Any oxidant present in the sodium pertechnetate Tc 99m may adversely affect the radiolabeling efficiency.
- Elute the Tc 99m generator according to the manufacturer’s instructions.
- Use only eluate from a Tc 99m generator which was eluted within the previous 24 hours.
- Prepare the technetium Tc99m exametazime with eluate that is not more than 2 hours old.
- Add 370 MBq up to 2000 MBq (10 mCi up to 54 mCi) sodium pertechnetate Tc 99m to Drax Exametazime vial.
- Before reconstitution, add up to 5 mL preservative-free, non-bacteriostatic Sodium Chloride Injection USP (0.9%) to the generator eluate to achieve a radioactive concentration no greater than 74-370 MBq/mL (2-10 mCi/mL).
- Measure the radioactivity and record as item (1) on the Technetium Tc 99m Exametazime Labeling Efficiency Worksheet.
- Use a sample for Quality Control [see Dosage and Administration (2.8)].
- Maintain reconstituted product at 20°C - 25°C (68°F - 77°F).
- Use for WBC labeling within 30 minutes.
- Discard any unused material according to local radiation safety procedures.
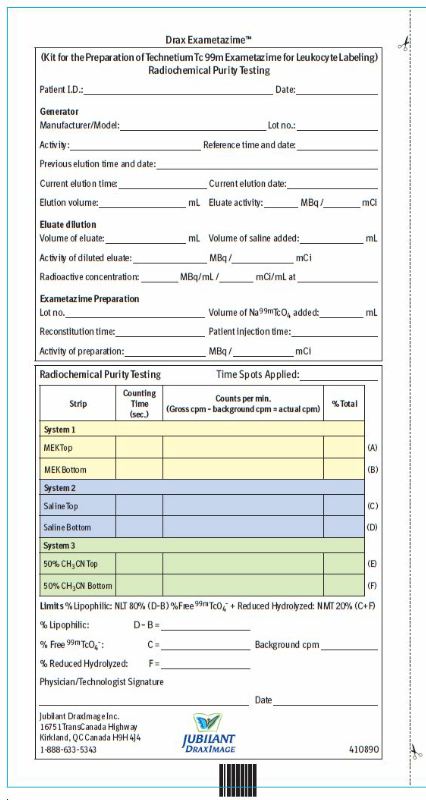
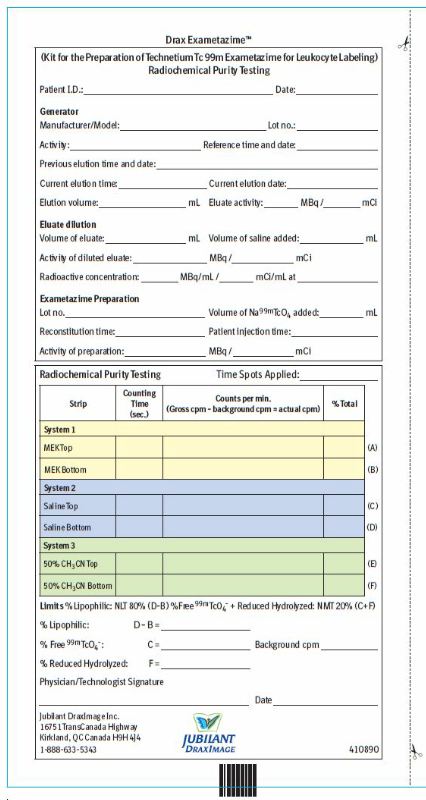
2.8 Radiochemical Purity Testing - Quality control of Tc 99m Exametazime
Obtain the Following Materials:
SG ITLC strips 6 cm x 0.7 cm
Whatman Grade 31ET chromatographic paper strip 6 cm x 0.7 cm
MEK (methyl ethyl ketone [butanone]) (99.9 + % HPLC Grade)
0.9% aqueous sodium chloride (non-bacteriostatic)
50% aqueous acetonitrile (99.9 + % HPLC Grade)
Glass test tubes (12 x 75 mm) with covers
1 mL syringes with 25-gauge needles.
Collimated radiation detector.- Perform radiochemical purity testing of technetium Tc 99m exametazime before leukocyte labeling and within 2 minutes of reconstitution.
- This entire radiochemical purity testing procedure takes approximately 15 minutes.
- A combination of 3 chromatographic systems is necessary for the complete definition of the radiochemical composition of the injection.
- System 1: methyl ethyl ketone (MEK) + SG ITLC strip
- System 2: 0.9% non-bacteriostatic sodium chloride solution + SG ITLC strip
- System 3: 50% acetonitrile solution + Whatman 31ET paper strip
- Three potential radiochemical impurities may be present in the prepared injection of the lipophilic Tc 99m exametazime complex:
- secondary Tc 99m exametazime complex
- free Tc 99m pertechnetate
- reduced-hydrolyzed Tc 99m
Method
1. Prepare three chromatographic systems using 12 mm × 75 mm chromatographic tubes with the following solvents (identify the solvent in each tube):
System 1- 0.3 mL of fresh methyl ethyl ketone (MEK),
System 2 - 0.9% non-bacteriostatic sodium chloride solution,
System 3 - 50% acetonitrile solution, prepared with non-bacteriostatic water
2. Apply 5 µL of freshly prepared Tc 99m exametazime solution (within 2 minutes of reconstitution) about 1 cm from the bottom of three strips: two 6cm × 0.7cm instant thin-layer chromatographic strips and one 6 cm × 0.7cm strip of chromatographic paper. Do not allow to dry.
3. Place one SG ITLC strip into the MEK tube (System 1), the second SG ITLC strip into the saline tube (System 2) and the Whatman 31ET paper strip into the 50% acetonitrile tube (System 3). Make sure strips are not adhering to the sides of the tube.
4. Allow the chromatograms to develop until the solvent front has moved to the top of the strips. Remove the strips from the tubes, and allow the solvents to evaporate.
5. Determine the radioactive distribution by scanning the strip sections, using a suitable collimated radiation detector.Chromatogram Interpretation
6. Using the Radiochemical Purity Worksheet, record the following counts:System 1 (SG ITLC: MEK [butanone]) Migrate at Rf 0.8-1 Lipophilic Tc 99m exametazime complex and Tc 99m pertechnetate Origin Secondary Tc 99m exametazime complex and reduced-hydrolyzed Tc 99m. System 2 (SG ITLC: 0.9% sodium chloride) Migrate at Rf 0.8-1 Tc 99m pertechnetate Origin Lipophilic Tc 99m exametazime complex, secondary Tc 99m exametazime complex and reduced- hydrolyzed Tc 99m System 3 (Whatman 31ET: 50% aqueous acetonitrile) Migrate at Rf 0.8-1 Lipophilic Tc 99m exametazime complex, secondary Tc 99m exametazime complex and Tc 99m pertechnetate Origin Reduced-hydrolyzed Tc 99m 7. Determine and record on the Radiochemical Purity Worksheet:
% at the origin of saline strip (D)
% at the origin of MEK strip (B)
% at the solvent front of saline strip (C) [% Tc 99m pertechnetate]
% at the origin of Whatman 31ET paper strip (F) [% reduced-hydrolyzed Tc 99m]8. Calculate the radiochemical purity:
% lipophilic exametazime complex = % at the origin of saline strip (D) – % at the origin of MEK strip (B)9. Do not use if radiochemical purity of Lipophilic Tc 99m Exametazime is less than 80%
2.9 Radiation Dosimetry
Based on human data, the radiation absorbed doses in an adult patient from an intravenous injection of Tc 99m labeled leukocytes have been estimated and are provided in Table 1.
Table 1 Tc 99m Exametazime Labeled Leukocytes Organ Absorbed dose per unit activity administered Absorbed dose per unit activity administered (microGy/MBq) (mrad/mCi) Spleen 88 327 Red marrow 15 54 Liver 13 48 Bone surfaces 8.4 31 Urinary bladder 8.2 30 Lungs 7.4 27 Stomach 6.7 25 Upper large intestine 5.5 20 Colon 4.5 17 Lower large intestine 3.3 12 Small intestine 2.5 9.3 Ovaries 1.6 6 Thyroid 1.3 4.8 Breast 0.9 3.3 Testes 0.8 3 Kidneys 0.3 1.1 Remaining organs 2.2 8.1 Effective dose per administered activity 7.5 microSv/MBq 28 mrem/mCi -
3 DOSAGE FORMS AND STRENGTHS
Drax Exametazime is a kit containing five (5) single-dose vials. Each 10 mL, clear glass vial contains a non-radioactive lyophilized mixture of: 0.5 mg exametazime, 7.6 mcg stannous chloride dihydrate (minimum stannous tin 0.6 mcg; maximum total stannous and stannic tin 4 mcg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure.
When reconstituted with the technetium Tc 99m eluate, each vial will contain a clear, colorless, and foreign particles-free solution of 370 MBq up to 2000 MBq (10 mCi up to 54 mCi) [74 - 370 MBq / mL (2 - 10 mCi / mL)]. The radioactive solution produced will be used for leukocyte labeling before intravenous administration to the patient.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including serious signs and symptoms of anaphylaxis, following administration of Tc 99m labeled leukocytes prepared using Tc 99m exametazime have been reported. Always have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions.
5.2 Risk for Image Interpretation Error
The interpretation of images can be affected by the presence of other pathophysiological processes within and outside of the abdominal cavity such as: tumor, infarction, trauma, and other inflammatory conditions.
5.3 Radiation Exposure Risk
Technetium Tc 99m contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling and preparation reconstitution procedures to protect patients and health care workers from unintentional radiation exposure. Encourage patients to drink fluids and void as frequently as possible after administration [see Dosage and Administration (2.1,2.2)].
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling;
- Hypersensitivity reactions [see Warnings and Precautions (5.1)].
The following adverse reactions associated with the use of technetium Tc 99m exametazime have been identified in clinical trials or post-marketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular: transient blood pressure increase
- Skin and subcutaneous tissue disorders: rash, generalized erythema, urticaria, angioedema, pruritus.
- General disorders and administration site conditions: facial edema, fever, asthenic conditions (e.g., malaise, fatigue).
- Nervous system disorders: headache, dizziness, paraesthesia.
- Vascular disorders: flushing.
- Gastrointestinal disorders: nausea, vomiting.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with technetium Tc 99m exametazime use in pregnant women are insufficient to inform a drug associated risk for major birth defects and miscarriage. Technetium Tc 99m exametazime is transferred across the placenta [see Data]. Animal reproduction studies with technetium Tc 99m exametazime have not been conducted. However, all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering technetium Tc 99m exametazime administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from technetium Tc 99m exametazime and the gestational timing of exposure.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Data
Human Data
Limited published literature describes Tc-99m exametazime crossing the placental barrier and accumulating in the fetal liver. No adverse fetal effects or radiation-related risks have been identified for diagnostic procedures involving less than 50mGy, which represents less than 10mGy fetal doses.8.2 Lactation
Risk Summary
There are limited data available in the scientific literature on the presence of technetium Tc 99m exametazime in human milk. There no data available on the effects of technetium Tc 99m exametazime on the breastfed infant or the effects on milk production. Exposure of technetium Tc 99m exametazine to a breast fed infant can be minimized by temporary discontinuation of breastfeeding [see Clinical Considerations]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for technetium Tc 99m exametazime, any potential adverse effects on the breastfed child from technetium Tc 99m exametazime or from the underlying maternal condition.Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breast milk after the administration of technetium Tc 99m exametazime-labeled leukocytes for 12 to 24 hours, where the duration corresponds to the typical range of administered activity, 259 MBq to 925 MBq (7 mCi to 25 mCi).8.5 Geriatric Use
Clinical studies of Tc 99m exametazime did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Technetium Tc 99m exametazime is substantially excreted by the kidneys, so excretion is decreased / delayed and therefore radiation exposure is greater in patients with impaired renal function. A reduction in administered Tc 99m can be considered provided an adequate number of labeled WBCs are administered.
- 10 OVERDOSAGE
-
11 DESCRIPTION
11.1 Chemical Characteristics
Drax Exametazime (kit for the preparation of technetium Tc 99m exametazime for leukocyte labeling) prepares a radioactive diagnostic agent. Each single-dose vial contains a sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime, 7.6 mcg stannous chloride dihydrate (minimum stannous tin 0.6 mcg; maximum total stannous and stannic tin 4 mcg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
The chemical formula of exametazime is C13H28N4O2, with the following structural formula:
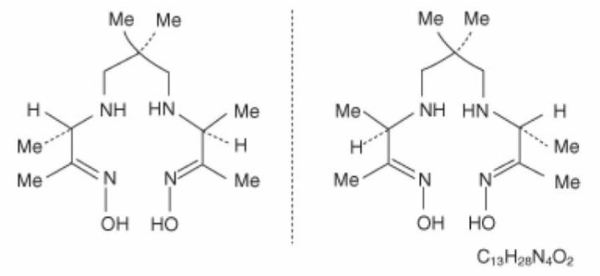
Prior to publication of the USAN, exametazime [also known as (RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime] was known as hexamethylpropylene amine oxime (HM-PAO). The name HM-PAO appears in many publications.
When Tc 99m pertechnetate in Sodium Chloride Injection, USP (0.9%) is added to Drax Exametazime vial, a Tc 99m complex of exametazime is formed.
11.2 Physical Characteristics
Tc 99m decays by isomeric transition with a physical half-life of 6 hours. Photons that are useful for imaging studies are listed in Table 2.
Table 2 Principal Radiation Emission Data - Tc 99m Radiation Mean %/Disintegration Mean Energy (keV) Gamma 2 88.5 140.5 11.3 External Radiation
The air-kerma-rate (exposure-rate) constant for technetium Tc 99m is 5.23 m2·pGy·(MBq)-1·s-1 [0.795 cm2·R·(mCi)-1·h-1]. The first half-value thickness of lead (Pb) for Tc 99m is 0.25 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 3. For example, the use of a 3 mm thickness of Pb will decrease the external radiation exposure by a factor of approximately 1,000.
Table 3 Radiation Attenuation by Lead Shielding Shield Thickness (Pb) mm Coefficient of Attenuation 0.25 0.5 1 10-1 2 10-2 3 10-3 4 10-4 5 10-5 To correct for physical decay of this radionuclide, the fractions that remain at selected intervals relative to the time of calibration are shown in Table 4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
When technetium Tc 99m pertechnetate is added to exametazime in the presence of stannous reductant, a lipophilic technetium Tc 99m complex is formed. This lipophilic complex is the active moiety. The lipophilic technetium Tc 99m exametazime complex is taken up and retained in leukocytes.
12.2 Pharmacodynamics
The relationship between technetium Tc 99m exametazime labeled WBC concentrations and successful imaging was not explored in clinical trials. GI activity is typically not seen through the initial four (4) hours post-injection. By four (4) hours, the hepatobiliary excretion of the Tc 99m exametazime allows tracer (Tc 99m) accumulation in the hepatobiliary system and, hence bowel activity.
12.3 Pharmacokinetics
Distribution
During the first hour following the injection, radioactivity is seen in the lungs, liver, gall bladder, spleen, blood pool, bone marrow, kidneys, and bladder. Over the first 1-6 hours, the Tc 99m is visualized in the bowel. At 24 hours post-injection substantial colonic activity is seen. The normal areas visualized in earlier scans are still visible.
Elimination
Excretion
Tc 99m excretion occurs via urine and feces. -
14 CLINICAL STUDIES
Two clinical trials of technetium Tc 99m exametazime were performed in a total of 88 patients who had suspected intra-abdominal infection or inflammation. Subjects received both Tc 99m labeled leukocytes and a radiolabeled comparator. Images were obtained at 2 and 30 minutes and at 2, 4 and 24 hours. In two other clinical trials, a total of 127 patients with suspected abdominal inflammation or infection received Tc 99m labeled leukocytes. Imaging was at 24 hours in one study and at 1, 3 and 24 hours in the other. In all four studies images were blindly evaluated and the findings were confirmed by surgery, biopsy or other clinical data.
Based on the above 4 studies, between 2 to 4 hours Tc 99m labeled leukocytes had 95-100% sensitivity and 62-85% specificity. In all studies the false positive and false negatives relate to the bowel background, the location of the site of infection/inflammation and whether or not it is contiguous with the bowel. Images obtained at 24 hours can be unreliable because of a high bowel background.
The interpretation of the images could also be affected by the presence of tumors, infarction and peritonitis [See Warning and Precautions (5.2)]. Liver abscess may be missed in planar imaging because of the bowel background.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Drax Exametazime kit (NDC 65174-200-05) comprises:
- 5 Single-dose vials (0.5 mg/vial). Each vial contains a non-radioactive sterile, non-pyrogenic lyophilized mixture of: 0.5 mg of exametazime, 7.6 mcg stannous chloride dihydrate, and 4.5 mg sodium chloride (NDC 65174-200-01);
- 10 Radiation Labels/Radiolabeled Leukocytes Labels/Lead Pot Labels ;
- 5 Labeling Efficiency/Radiochemical Purity Testing Worksheets;
- 1 Leukocyte Labeling Schematic;
- 1 Package Insert.
Sodium Pertechnetate Tc 99m is not part of Drax Exametazime kit. Before reconstitution and radiolabeling with Tc 99m, the contents of the kit are not radioactive.
16.2 Storage and Handling
Store Drax Exametazime kit at 15°C - 25°C (59°F - 77°F).
Drax Exametazime is for distribution to and use by persons licensed authorized by the U.S. Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State. Store and dispose of technetium Tc 99m exametazime in compliance with the appropriate regulations of the government agency authorized to license the use of this radionuclide. -
17 PATIENT COUNSELING INFORMATION
Administration Instructions:
- Advise patients to hydrate after administration of technetium Tc 99m exametazime labeled leukocytes and to void frequently to minimize radiation dose [see Dosage and Administration (2.2)].
Pregnancy
- Advise pregnant women of the risk of fetal exposure to radiation doses if they undergo a radionuclide procedure [see Use in Specific Populations (8.1)].
Lactation
- Advise lactating women that exposure of the infant to technetium Tc 99m through breast milk can be minimized if breastfeeding is interrupted when technetium Tc99m exametazime labeled leukocytes are administered. Advise a lactating woman to pump and discard breast milk for 12 to 24 hours based on injected dose [see Use in Specific Populations (8.2)].
Manufactured for:
Jubilant DraxImage Inc., Kirkland, Quebec, Canada, H9H 4J4.
Art rev.: 1.0 -
Carton Label

Rx only
For Reconstitution and Radiolabelling Instructions:See package insert.Important: For intravenous use as directed.
Use appropriate radiation shielding.
Use only for radiolabeling of leukocytes after reconstitution with technetium Tc 99m.Storage Conditions: Store the kit at 15°C-25°C (59°F-77°F) up to the expiry date. After reconsitution with technetium Tc 99m, store at 20°C-25°C (68°F-77°F) and use for labeling leukocytes within 30 minutes.
NDC 65174-200-05
5 single dose vialsDrax Exametazime
Kit for the Preparation of Technetium Tc 99m
Exametazime for Leukocyte Labeling
0.5 mg/vialManufactured for
Jubilant DraxImage Inc.
Kirkland, Quebec H9H 4J4 CanadaContents: 5 single-dose vials each containing a sterile and non-pyrogenic lyophilized mixture of:
- 0.5 mg exametazime
- 7.6 mcg stannous chloride dihydrate (minimum stannous tin 0.6 mcg; maximum total stannous and stannic tin 4mcg per vial)
- 4.5 mg sodium chloride
10 Radiation Labels/Radiolabeled Leukocyte Labels/Lead Pot Labels
5 Labeling Efficiency/Radiochemical Purity Testing Worksheets
1 Leukocyte Labeling Schematic
1 Package InsertMade in Canada
Vial Label

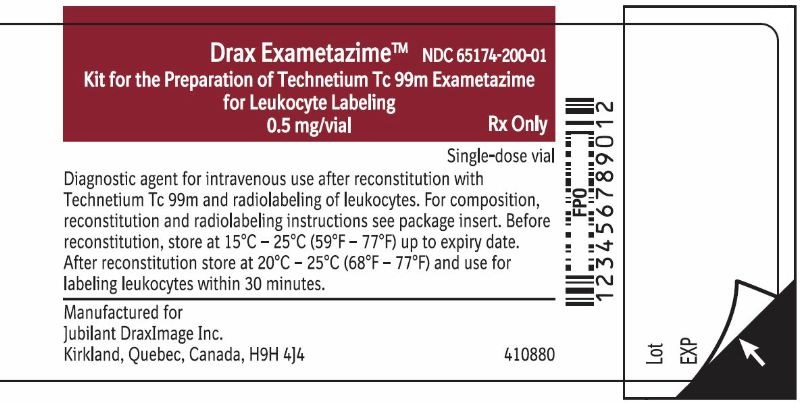
Drax Exametazime
Kit for the Preparation of Technetium Tc 99m Exametazime
for Leukocyte Labeling
0.5 mg/vialNDC 65174-200-01
Rx OnlySingle-dose vial
Diagnostic agent for intravenous use after reconstitution with Technetium Tc 99m and radiolabeling of leukocytes. For composition, reconstitution and radiolabeling instructions see package insert. Before reconstitution, store at 15°C-25°C (59°F-77°F) up to expirty date. After reconstitution store at 20°C-25°C (68°F-77°F) and use for labeling leukocytes within 30 minutes.Manufactured for
Jubilant DraxImage Inc.
Kirkland, Quebec, Canada H9H 4J4Contains 0.5 mg of exametazime per vial, 7.6 mcg stannous chloride dihydrate (minimum stannous tin 0.6 mcg; maximum total stannous and stannic tin 4 mcg per vial), and 4.5 mg sodium chloride.
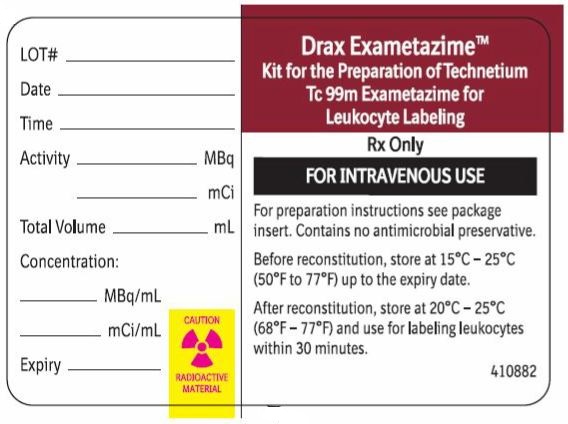
Radiation Label
Drax Exametazime
Kit for the Preparation of Technetium
Tc 99m Exametazime for Leukocyte Labeling
Rx Only
FOR INTRAVENOUS USEFor preparation instructions see package insert. Contains no antimicrobial preservative.
Before reconstitution, store at 15°C-25°C (59°F-77°F) up to expiry date.
After reconstitution store at 20°C-25°C (68°F-77°F) and use for labeling leukocytes within 30 minutes.Radiolabeled Leukocyte Lead Pot Label

Drax Exametazime
Kit for the Preparation of Technetium
Tc 99m Exametazime for Leukocyte Labeling
Rx Only
FOR INTRAVENOUS USERadiolabled Leukocytes
For preparation instructions see package insert.
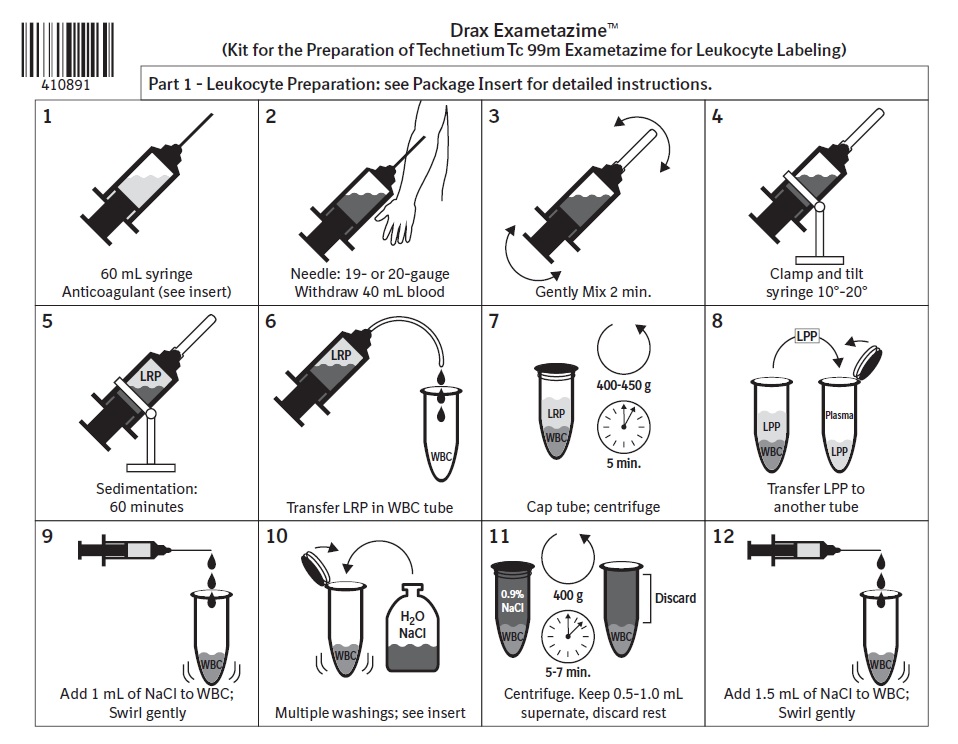
Labeling Schematic
Drax Exametazime (Kit for the Preparation of Technetium Tc 99m Exametazime for Leukocyte Labeling)
Leukocyte Preparation: see Package Insert for detailed instructions.
- 60 mL syringe
Anticoagulant (see insert) - Needle: 19- or 20-gauge
Withdraw 40mL blood - Gently Mix 2 min.
- Clamp and tilt syringe 10°-20°
- Sedimentation: 60 minutes
- Transfer LRP in WBC tube
- Cap tube; centrifuge
- Transfer LPP to another tube
- Add 1 mL of NaCl to WBC;
Swirl gently - Multiple washings; see insert
- Centrifuge. Keep 0.5-1.0 mL supernate, discard rest
- Add 1.5 mL of NaCl to WBC;
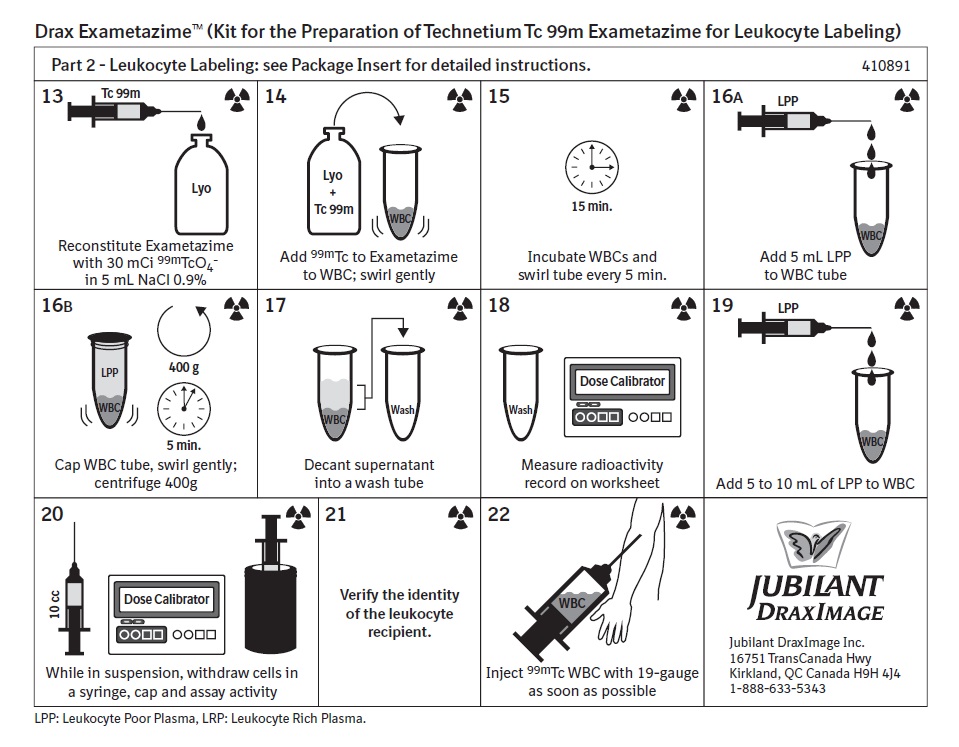
Swirl gently - Reconstitute Exametazime with 30mCi99mTcO4- in 5 mL NaCl 0.9%
- Add 99mTc to Exametazime to WBC; swirl gently
- Incubate WBCs and swirl tube every 5 min.
- Add 5 mL LPP to WBC tube
Cap WBC tube, swirl gently; centrifuge 400g - Decant supernatant into a wash tube
- Measure radioactivity record on worksheet
- Add 5 to 10 mL of LPP to WBC
- While in suspension, withdraw cells in a syringe, cap and assay activity
- Verify the identity of the leukocyte recipient.
- Inject 99mTc WBC with 19-gauge as soon as possible
Jubilant DraxImage Inc.
16751 TransCanada Hwy
Kirkland, QC Canada H9H 4J4
1-888-633-5343LPP: Leukocyte Poor Plasma, LRP: Leukocyte Rich Plasma
RCP Efficiency Worksheets

-
INGREDIENTS AND APPEARANCE
DRAX EXAMETAZIME
kit for the preparation of technetium tc 99m exametazime for leukocyte labeling injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65174-200 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TECHNETIUM TC-99M EXAMETAZIME (UNII: 3B744AG22N) (TECHNETIUM TC-99M EXAMETAZIME - UNII:3B744AG22N) EXAMETAZIME 0.5 mg Inactive Ingredients Ingredient Name Strength STANNOUS CHLORIDE (UNII: 1BQV3749L5) SODIUM CHLORIDE (UNII: 451W47IQ8X) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65174-200-05 5 in 1 CARTON 08/17/2017 1 NDC:65174-200-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208870 08/17/2017 Labeler - Jubilant DraxImage Inc., dba Jubilant Radiopharma (243604761) Establishment Name Address ID/FEI Business Operations Jubilant DraxImage Inc., dba Jubilant Radiopharma 243604761 manufacture(65174-200) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier General Partnership 246762764 manufacture(65174-200)