Label: SODIUM BICARBONATE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 50509-100-01, 50509-100-02, 50509-100-03, 50509-100-04 - Packager: Onpharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 31, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
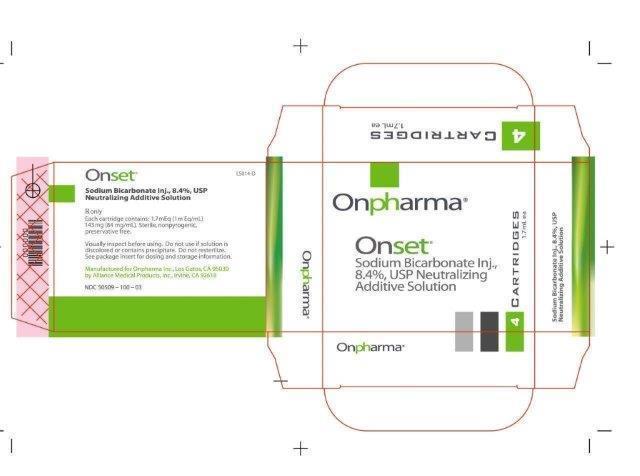
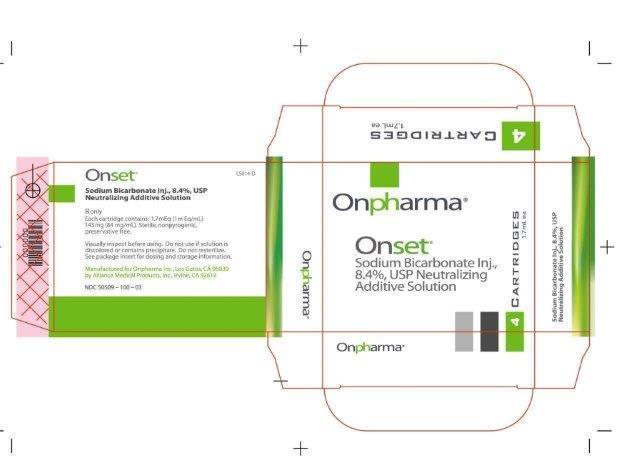
Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution is a sterile, nonpyrogenic, solution of sodium bicarbonate (NaHCO3) in Water for Injection. It is added to an appropriate local anesthetic as a neutralizing agent immediately prior to administration.
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for single-use. pH is adjusted with carbon dioxide. Per the USP monograph for Sodium Bicarbonate Inj., pH is between 7.0 and 8.5. Osmolar concentration is 2 mOsmol/mL (calc.).
Sodium bicarbonate, 84 mg is equal to one milliequivalent each of Na+ and HCO3-.
Sodium Bicarbonate, USP is chemically designated as NaHC03, a white crystalline powder soluble in water. Sodium bicarbonate in water dissociates to provide sodium (Na+) and bicarbonate (HCO3-) ions.
Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate (HCO3-) is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mEq/liter. Bicarbonate anion is considered “labile” since at a proper concentration of hydrogen ion (H+) it may be converted to carbonic acid (H2CO3) and thence to its volatile form, carbon dioxide (CO2) excreted by the lung. Normally a ratio of 1:20 (carbonic acid; bicarbonate) is present in the extracellular fluid. In a healthy adult with normal kidney function, practically all the glomerular filtered bicarbonate ion is reabsorbed; less than 1% is excreted in the urine.
Non-neutral parenteral solutions with a low (acidic) pH are known to cause chemical irritation of tissues.
- INDICATIONS AND USAGE:
-
DOSAGE & ADMINISTRATION
The practitioner should choose a volume of Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution to be mixed with Lidocaine w/ Epinephrine in a ratio of 1:10 (local anesthetic solution to sodium bicarbonate solution).
The below table provides a mixing guide showing for convenience the volumes of 8.4% Sodium Bicarbonate Neutralizing Additive Solution to be added to the commercial preparations of Lidocaine with Epinephrine in order to achieve a mixed ratio of 10:1.10:1 Anesthetic-to-Bicarbonate Solution Ratio Mixing Guide for 10:1
Volume (mL), Lidocaine w/ Epinephrine (container type) Volume (mL), 8.4% Sodium Bicarbonate Solution 1.8 mL (cartridge) 0.18 mL 20 mL (Vial) 2.0 mL 30 mL (Vial) 3.0 mL 50 mL (Vial) 5.0 m - CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
Administer local anesthetic solution immediately after combining with Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution.
When combining local anesthetic solution with Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution, use aseptic technique, mix thoroughly, and do not store.
Do not use unless Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution is clear, colorless, and free of particulates or cloudiness, and the container or seal is intact. Do not use if the inner or outer packaging are damaged. Discard unused portion.Do not use local anesthetic combined with Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution unless the combined solution is clear, colorless, and free of particulates or cloudiness.
Parenteral drug products should be inspected visually for particulate matter, cloudiness and discoloration prior to administration, whenever solution and container permit.
- Drug Interactions
-
Pregnancy Category C
Animal reproduction studies have not been conducted in which Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution was evaluated. Animal reproduction studies have not been conducted in which Lidocaine w/ Epinephrine that has been pH adjusted by the addition of Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution was evaluated.
It is not known whether Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution can cause fetal harm when administered to a pregnant woman or whether it can affect reproduction capacity. It is not known whether Lidocaine w/ Epinephrine that has been pH adjusted by the addition of Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution can cause fetal harm when administered to a pregnant woman or whether it can affect reproduction capacity.
- ADVERSE REACTIONS:
-
OVERDOSAGE:
Adding a volume of Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution to Lidocaine w/ Epinephrine solution such that the pH of the Lidocaine w/ Epinephrine is raised above physiologic pH may cause anesthetic to precipitate out of solution, reducing the clinical effectiveness of the anesthetic. See, e.g., Mulroy MF, Regional Anesthesia, An Illustrated Procedural Guide, 3rd Ed. 2002 (Lippincott Williams and Wilkins, Philadelphia, PA). In addition, solutions that contain precipitate should not be injected.
Adding a volume of Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution to Lidocaine w/ Epinephrine solution such that the pH of the Lidocaine w/ Epinephrine is raised well above (7.8) physiologic pH may cause tissue irritation when the solution is injected. See Whitcomb M, et al, A Prospective Randomized, Double Blind Study of the Anesthetic Efficacy of Sodium Bicarbonate Buffered 2% Lidocaine with 1:100,000 Epinephrine in Inferior Alveolar Nerve Blocks, Anesth Prog, vol 57, p 59 (2010). - HOW SUPPLIED:
- STORAGE AND HANDLING
-
REFERENCES, INDICATIONS AND USAGE:
Barash PG, Cullen BF, Stoelting RK, Clinical Anesthesia (4th Ed. 2001 Lippincott Williams and Wilken).
Bhatt H, Powell KJ, Jean DA, First Aid for the Anesthesiology Boards, An Insider's Guide (2011, McGraw-Hill Medical).
Cepeda MS, Tzortzopoulou A, Thackrey M, Hudcova J, Arora Gandhi P, Schumann R., Adjusting the pH of lidocaine for reducing pain on injection. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No.: CD006581.
Chu LF, Clinical Anesthesiology Board Review(2005, McGraw-Hill Medical).
Malamed SF,Handbook of Local Anesthesiology(5th Ed. 2004, Elsevier Mosby).
Miller RD, Miller's Anesthesia (6th Ed. 2004).
Stoelting RK, Miller RD,Basics of Anesthesia(5th Ed. 2007, Churchill Livingstone Elsevier).REFERENCES, DOSAGE:
Christoph RA, Buchanan L, Begalla K, Schwartz S, Pain reduction in local anesthetic administration through pH buffering, Annals of Emergency Medicine, vol 17, no 2, p 117 (1988).
Martin AJ, pH-adjustment and discomfort caused by the intradermal injection of lignocaine, Anaesthesia, vol 45, p 975 (1990).
Masters JE, Randomised control trial of pH buffered lignocaine with adrenaline in outpatient operations, British Journal of Plastic Surgery, vol 51, p 385 (1998).
McKay W, Morris R, Mushlin P, Sodium bicarbonate attenuates pain on skin infiltration with or without epinephrine, Anesthesia and Analgesia, vol 66, p 572 (1987).
Narvaez J, Wessels I, Bacon G, Chin VR, Baqai WK, Zimmerman GJ, Prospective randomized evaluation of short-term complications when using buffered or unbuffered lidocaine 1% with epinephrine for blepharoplasty surgery, Ophthalmic Plastic and Reconstructive Surgery, vol 26, no1, p 33 (2010).
Talu H, Elibol O, Yanyali A, Karabas L, Alp B, Caglar Y, Effect of warming and buffering lidocaine on pain during facial anesthesia, Annals of Ophthalmology, vol 33, no 1, p 43 (2001).
- SPL UNCLASSIFIED SECTION
- 1.7 mL Vial Carton Image:
- 1.7 mL Vial Label Image:
-
INGREDIENTS AND APPEARANCE
SODIUM BICARBONATE
sodium bicarbonate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50509-100 Route of Administration PARENTERAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 84 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50509-100-02 4 in 1 CARTON 1 NDC:50509-100-01 2.7 mL in 1 CARTRIDGE 2 NDC:50509-100-04 4 in 1 CARTON 2 NDC:50509-100-03 1.7 mL in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/22/2010 Labeler - Onpharma, Inc. (011848357) Establishment Name Address ID/FEI Business Operations WuXi AppTec, Inc. 136584468 manufacture(50509-100) Establishment Name Address ID/FEI Business Operations Avrio Biopharmaceuticals, LLC 829688899 manufacture(50509-100) Establishment Name Address ID/FEI Business Operations Alliance Medical Products, Inc 102688657 manufacture(50509-100)