TORSEMIDE
- torsemide injection, solution
General Injectables and Vaccines, Inc.
----------
DESCRIPTION
Torsemide Injection is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-
[(4-rn-toluidino-3-pyridyl) sulfonyl]urea and its structural formula is:
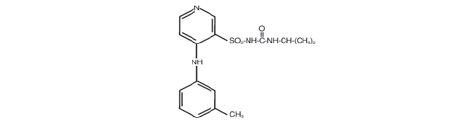
Its molecular formula is C,sH;!ON4 0 3S, its pKa is 6.42, and its molecular weight is 348.42.
Torsemide is a white to off-white c rys ta lline powder. Torsemide vials for intravenous injection contain a
sterile solution of torsemide (10 mglmL), polyethylene g lycal-400, tromethamine, and sodium hydroxide
(as needed to adjust p H ) in water for injection.
CLINICAL PHARMACOLOGY
Mechanism of Action
Micropuncture studies in anima ls have shown that torsemide acts from within the lumen of the thick
ascending portion of the loop of Henle, where it inhibits the Na'JK'/2CI'-carrier system. Clinical
pharmacology studies have confirmed this site of action in humans, and effects in other segments of
the nephron have not been demonstrated. Diuretic activity thus correlates better with the rate of drug
excretion in the urine than with the concentration in the blood.
Torsemide increases the urinary excretion of sodium, chloride, and water, but it does not significantly
alterglomerular filtration rate, renal plasma flow, or acid-base balance.
Pharmacokinetics and Metabolism
The volume of distribution of torsemide is 12 liters to 15 liters in normal adults or in patients with mild
to moderate renal failure or congestive heart failure. In patients with hepatic cirrhosis, the volume of
distribution is approximately doubled.
In normal subjects the elimination half- life of torsemide is approximately 3.5 hours. Torsemide is cleared
from the circulation by both hepatic metabolism (approximately 80% of total clearance) and excretion
into the urine (approximately 20% of total clearance in patients w ith normal renal function). The major
metabolite in humans is the carboxylic acid derivative, which is biologically inactive. Two of the lesser
metabolites possess some diuretic activity, but for practical purposes metabolism terminates the action
of the drug.
Because torsemide is extensively bound to plasma protein (> 990/0), very little enters tubular urine via
glomerular filtration. Most renal clearance of torsemide occurs via active secretion of the drug by the
proximal tubules into tubular urine.
In patients with decompensated congestive heart failure, hepatic and renal clearance are both reduced,
probably because of hepatic congestion and decreased renal plasma flow, respectively. The total
clearance of torsemide is approximately 50% of that seen in healthy volunteers, and the plasma half-life
and AUG a re correspondingly increased. Because of reduced renal clearance, a smaller fraction of any
given dose is delivered to the intraluminal site of action, so at any given dose there is less natriuresis in
patients with congestive heart failure than in normal subjects.
In patients with renal failure, renal clearance of torsemide is markedly decreased but total plasma
clearance is not significantly altered. A smaller fraction of the administered dose is delivered to the
intraluminal site of action, and the natriuretic action of any given dose of diuretic is reduced. A diuretic
response in renal failure may still be achieved if patients are given higher doses. The total plasma
clearance and elimination half-life of torsemide remain normal under the conditions of impaired renal
function because metabolic elimination by the liver remains intact.
In patients with hepatic cirrhosis, the volume of distribution, plasma half-life, and renal clearance are all
increased, but total clearance is unchanged.
The pharmacokinetic profile of torsemide in healthy elderly subjects is similar to that in young subjects
except for a decrease in renal clearance related to the decline in renal function that commonly occurs
with aging. However, total plasma clearance and elimination half-life remain unchanged.
Clinical Effects
The diuretic effect s of torsemide begin within 10 minutes of intravenous dosing and peak within the first
hour. With intravenous administration diuresis lasts about 6 to 8 h ours. In healthy subjects given single
doses, the dose-response relationship for sodium excretion is linear over the dose range o f 2.5 mg to 20
mg. The increase in potassium excretion is negligible after a single dose of up to 10 mg and only slight
(5 mEq to 15 mEq) after a single dose o f 20 mg.
Congestive Heart Failure
Torsemide has been studied in controlled trials in patients with New York Heart Association Class II to
Class IV congestive heart failure . Patients who received 10 mg to 20 mg o f daily torsemide in these
studies achieved significantly greater reductions in weight and edema than did patients who received
placebo.
Nonanuric Renal Failure
In single-dose studies in patients with nonanuric renal failure, high doses of torsemide (20 mg to 200
mg) caused marked increases in water and sodium excretion . In patients with nonanuric renal failure,
severe enough to require hemodialys is, chronic treatment with up to 200 mg of daily torsemide has
not been shown to change steady-state fluid retention . When patients in a study of acute renal failure
received total daily doses of 520 mg to 1200 m g o f to rsemide, 1 9% experienced seizures. Ninety-six
patients were treated in this study; 6/32 treated with torsemide experienced seizures, 6/32 treated
with comparably high doses of furosemide experienced seizures, and 1/32 treated with placebo
experienced a seizure.
Hepatic Cirrhosis
When given with aldosterone antagonists, torsemide also caused increases in sodium and fluid
excretion in patients with edema or ascites due to hepatic cirrhosis. Urinary sodium excretion rate
relative to the urinary excretion rate of torsemide is less in cirrhotic patients than in healthy subjects
(possibly because of the hyperaldosteronism and resultant sodium retention that are characteristic of
portal hypertension and ascites). However, because of the increased renal clearance of torsemide in
patients with hepatic cirrhosis, these factors tend to balance each other, and the result is an overall
natriuretic response that is similar to that seen in healthy subjects. Chronic use of any diuretic in hepatic
disease has not been studied in adequate and well-controlled trials.
Essential Hypertension
In patients with essential hypertenSion, torsemide has been shown in controlled studies to lower blood
pressure when administered once a day at doses of 5 mg to 10 mg. The antihypertensive effect is near
maximal after 4 to 6 weeks of treatment, but it may continue to increase for up to 12 weeks. Systolic and
diastolicsupine and standing blood pressures are all reduced. There is no significant orthostatic effect,
and there is only a minimal peak-trough difference in blood pressure reduction .
The antihypertensive effects of torsemide are, like those of other diuretics, on the average greater in
black patients (a low-renin population) than in non black patients.
When torsemide is first administered, daily urinary sodium excretion increases for a t least a week. With
chronic administration, however, daily sodium loss comes into balance with dietary sodium intake. If
the administration of torsemide is suddenly stopped, blood pressure returns to pretreatment levels over
several days, without over shoot.
Torsemide has been administered together with j3-adrenergic blocking agents, ACE inhibitors, and
calcium-channel blockers. Adverse drug inte rac tio n s have n o t been observed, and special dosage
adjustment has not been necessary.
Torsemide Injection is indicated for the treatment of edema associated with congestive heart failure,
renal disease, or hepatic disease. Use of torsemide has been found to be effective for the treatment of
edema associated w th chronic renal failure. Chronic use of any diuretic in hepatic disease has not been
studied in adequate and well-controlled trials.
Torsemide Injection is indicated when a rapid onset of diuresis is desired or when oral administration
is impractical.
Torsemide Injection is indicated for the treatment of hypertension alone or in combination with other
antihypertensive agents.
CONTRAINDICATIONS
Torsemide Injection is contraindicated in patients with known hypersensitivity to torsemide or to
sulfonylureas.
Torsemide Injection is contraindicated in patients who are anuric.
WARNINGS
Hepatic Disease With Cirrhosis and Ascites
Torsemide should be used with caution in patients with hepatic disease with cirrhosis and ascites, since
sudden alterations of fluid and electrolyte balance may precipitate hepatic coma. In these patients,
diuresis with torsemide (or any other diuretic) is best initiated in the hospital. To prevent hypokalemia
and metabolic alkalosis, analdosteroneantagonist or potassium-sparing drug should be used
concomitantly with torsemide.
Ototoxicity
Tinnitus and hearing loss (usually reversible) have been observed after rapid intravenous injection of
other loop diuretics and have also been observed after oral Torsemide. It is not certain that these events
were attributable to torsemide . Ototoxicity has also been seen in animal studies when very high plasma
levels of torsemide were induced. Administered intravenously, torsemide should be injected slowly over
2 minutes, and single doses should not exceed 200 mg.
Volume and Electrolyte Depletion
Patients receiving diuretics should be observed for clinical evidence of electrolyteim balance,
hypovolemia, orprerenal azotemia . Symptoms of these disturbances may include one or more of the
following: dryness of the mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains
or cramps, muscular fatigue , hypotension , oliguria, tachycardia, nausea, and vomiting. Excessive
diuresis may cause dehydration, blood-volume reduction , and possibly thrombosis and embolism,
especially in elderly patients. In patients who develop fluid and electrolyte imbalances, hypovolemia,
orprerenal azotemia, the observed laboratory changes may include hyper-orhypon atremia, hyper -or
hypochloremia, hyper- or hypokalemia, acid-base abnormalities, and increased blood urea nitrogen
(BUN). If any of these occur, to rsemide should be discontinued until the situation is corrected; torsemide
may be restarted at a lower dose.
In controlled studies in the United States, torsemide was administered to hypertensive patients at doses
o f 5 mg or 10 mg daily. Afte r 6 weeks at these doses, the mean decrease in serum potassium was
approximately 0.1 mEq/L. The percentage of patients who had a serum potassium level below 3.5 m Eq/L
at any time during the studies was essentially the same in patients who received torsemide ( 1.5%) as in
those who received placebo (3%) . In patients followed for 1 year, there was no further change in mean
serum potassium levels. In patients with congestive heart failure, hepatic cirrhosis, orrena l disease
treated with torsemide at doses higher than those studied in United States a ntihypertensive trials,
hypokalemia was observed with greater frequency, in a dose-related manner.
In patients with cardiovascular disease, especially those receiving digitalis glycosides, diuretic-induced
hypokelemia may be a risk factor for the development of arrhythmias. The risk of hypokalemia is
greatest in patients with cirrhosis of the liver, in patients experiencing a brisk diuresis, inpatients who
are receiving inadequate oral intake of electrolytes, and in patients receiving concomitant therapy with
corticosteroids or ACTH.
Periodic monitoring of serum potassium and other electrolytes is advised in patients treated with
torsemide.
PRECAUTIONS
Laboratory Values
Potassium
See WARNINGS.
Calcium
Single doses of torsemide increased the urinary excretion of calcium by normal subjects, but serum
calcium levels were slightly increased in 4 to 6 week hypertension trials . In a long-term study of patients
with congestive heart failure, the average 1 year change in serum calcium was a decrease of 0 .1 mgJdL
(0.02 mmoIlL). Among 426 patients treated with torsemide for an average o f 11 months, hypocalcemia
was not reported as an adverse event.
Magnesium
Single doses of torsemide caused healthy volunteers to increase the ir urinary excretion of magnesium,
but serum magnesium levels were slightly increased in 4 to 6 week hypertension trials. In long-term
hypertension studies, the average 1 year change in serum magnesium was an increase of 0.03 mgJdL
(0.01 mmoIlL). Among 426 patients treated with torsemide for an average of 11 months, one case of
hypomagnesemia (1 .3 m gJdL [0.53 mmollLJ) was reported as an adverse event .
In a tong- term clinical study of torsemide in patients with congestive heart failure, the estimated
annual change in serum magnesium was an increase of 0.2 mg/dL (0.08 mmoI/L), but these data are
confounded by the fact that many of these patients received magnesium supplements. In a 4 week
study in which magnesium supplementation was not given, the rate of occurrence of serum magnesium
levels below 1.7 mg/dL (0.7 mmollL) was 60/0 a nd 9% in the groups receiving 5 mg a nd 10 mg of
torsemide, respectively.
Blood Urea Nitrogen (BUN), C reatinin e a nd Uric Acid
Torsemide produces small dose-related increases in each of these laboratory values. In hypertensive
patients who received 10 mg o f torsemide daily for 6 weeks, the mean increase in blood urea nitrogen
was 1 .8 mg/dL (0.6 mmo IJL) , the mean increase in serum creatinine was 0.05 mg/dL (4 mmo t/ L) , and
the mean increase in serum uric acid was 1 .2 m gJdL (70 mmoIJL). Little further change occurred with
long-term treatment, and all changes reversed when treatment was discontinued.
Symptomatic gout has been reported in patients receiving torsemide, but its incidence has been similar
to that seen in patients receiving placebo.
Glucose
Hypertensive patients who received 10 m g of daily torsemide experienced a mean increase in serum
glucose concentration o f 5.5 mg/dL (0.3 mmot/L) after 6 weeks of therapy, with a further increase of
1 .8 mgldL (0.1 mmollL) during the subsequent year. In long-term studies in diabetics, mean fasting
glucose values were not significantly changed from baseline. Cases of hyperglycemia have been
reported but are uncommon.
Serum Lipids
In the controlled short-term hypertension studies in the United States, daily doses of 5 mg, 10 mg, and
20 mg of torsemide were associated with increases in total plasma cholesterol of 4, 4 , and 8 mg/dL (0.1
to 0.2 mmoIlL ), respectively. The changes subsided during chronic therapy.
In the same short- term hypertension studies, daily doses of 5 mg, 10 m g and 20 mg of torsemide were
associated with mean increases in plasma triglycerides of 16, 13, and 71 mg/dL (0. 15 to 0.8 mmoIlL) ,
respectively.
In long-term studies of 5 mg to 20 mg of torsemide daily, no clinically significant differences from
baseline lipid values were observed after 1 year of therapy.
Other
In long-term studies in hypertensive patients, torsemide has been associated with small mean
decreases in hemoglobin, hematocrit , and erythrocyte count and small mean increases in white blood
cell count, platelet count, and serum alkaline phosphatase. Although statistically significant, all of these
changes were medically inconsequential. No significant trends have been observed in any liver enzyme
tests other than alkaline phosphatase.
DRUG INTERACTIONS
In patients with essential hypertension, torsemide has been administered together with beta-blockers,
ACE inhibitors, and calcium-channel blockers, In patients with congestive heart failure, torsemide has
been administered together with digitalis glycosides, ACE inhibitors, and o rganic nitrates. None of these
combined uses was associated with new or unexpected adverse events.
Torsemide does not affect the protein binding of glyburide or of wartarin, the anticoagulant effect of
phenprocoumon (a related coumarin derivative), or the pharmacokinetics of digoxin or carvedilol (a
vasodilator/beta-blocker). In hea lthy subjects, coadministration of torsemide was associated with
significant reduction in the renal clearance of spironolactone, with corresponding increases in the AUC.
However, clinical experience indicates that dosage adjustment of either agent is not required ,
Because torsemide and salicylates compete fo r secretion by rena l tubules, patients receiving high
doses of salicylates may experience salicylate toxicity when torsemide is concomitantly administe red ,
Also, although possible inte ractions between torsemide and nonsteroidal anti-inflammatory agents
(including aspirin) have not been studied, coadministration of these agents with another loop diuretic
(furosemide) has occasionally been associated with renal dysfunction,
The natriuretic effect of torsemide (like that of many other diuretics) is partially inhibited by the
concomitant administration of indomethacin. This effect has been demonstrated for torsemide under
conditions of dietary sodium restriction (50 mEq/day) but not in the presence of normal sodium intake
(150 mEq/day).
The pharmacokinetic profile and diuretic activity of torsemide are not altered by cimetidine or
s pironolactone, Coadministration of digoxin is reported to increase the area under the curve for
torsemide by 50%, but dose adjustment of torsemide is not necessary.
Concomitant use of torsemide and cholestyramine has not been studied in humans but, in a study
in animals, coadministration of cholestyramine decreased the absorption of orally administered
torsemide. If torsemide and c holestyramine are used concomitantfy, simultaneous administration is
not recommended.
Coadministration of probenecid reduces secretion of torsemide into the proximal tubule and thereby
decreases the diuretic activity of torsemide.
Other diuretics are known to reduce the renal clearance of lithium, inducing a high risk of lithium
toxicity, so coadministration of lithium and diuretics should be undertaken with great caution, if at all.
Coadministration of lithium and torsemide has not been studied.
Other diuretics have been reported to increase the ototoxic potential of aminoglycoside antibiotics and
of ethacrynic acid, especially in the presence of impaired renal function. These potential interactions
with torsemide have not been studied.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No overall increase in tumor incidence was found when torsemide was given to rats and mice
throughout their lives at doses up to 9 mg/kg/day (rats) and 32 mg/kg/day (mice). On a body-weight
basis, these doses are 27 to 96 times a human dose of 20 mg; on a body-surface-area basis, they are
5 to 8 times this dose. In the rat study, the high-dose female group demonstrated renal tubular injury,
interstitial inflammation, and a statistically significant increase in renal adenomas and carcinomas. The
tumor incidence in this group was, however, not much higher than the incidence sometimes seen in
historical controls. Similar signs of chronic non· neoplastic renal injury have been reported in high·dose
animal studies of other diuretics such as furosemide and hydrochlorothiazide.
No mutagenic activity was detected in any of a variety of in vivo and in vitro tests of torsemide and
its major human metabolite. The tests included the Ames test in bacteria (with and without metabolic
activation), tests for chromosome aberrations and sister-chromatid exchanges in human lymphocytes,
tests for various nuclear anomalies in cells found in hamster and murine bone marrow, tests for
unscheduled DNA synthesis in mice and rats, and others.
In doses up to 25 mg/kg/day (75 times a human dose of 20 mg on a body-weight basis; 13 times this
dose on a body·surface·area basis), torsemide had no adverse effect on the reproductive performance
of male or female rats.
Pregnancy
Teratogenic Effects
Pregnancy Category B.
There was no fetotoxicity or teratogenicity in rats treated with up to 5 mg/kg/day of torsemide (on a
mg/kg basis, this is 15 times a human dose of 20 mg/day; on a mg/m2 basis, the animal dose is 10
times the human dose), or in rabbits, treated with 1.6 mg/kg/day (on a mg/kg basis, 5 times the human
dose of 20 mg/kg/day; on a mg/m2 basis, 1.7 times this dose). Fetal and maternal toxicity (decrease in
average body weight, increase in fetal resorption and delayed fetal ossification) occurred in rabbits and
rats given doses 4 (rabbits) and 5 (rats) times larger. Adequate and well-controlled studies have not
been carried out in pregnant women. Because animal reproduction studies are not always predictive of
human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
The effect of torsemide on labor and delivery is unknown.
Nursing Mothers
It is not known whether torsemide is excreted in human milk. Because many drugs are excreted in
human milk, caution should be exercised when torsemide is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Administration of another loop diuretic to severely premature infants with edema due to patent ductus
arteriosus and hyaline membrane disease has occasionally been associated with renal calcifications,
sometimes barely visible on X-ray but sometimes in staghorn form, filling the renal pelves. Some of
these calculi have been dissolved, and hypercalciuria has been reported to have decreased, when
chlorothiazide has been coadministered along with the loop diuretic. In other premature neonates with
hyaline membrane disease, another loop diuretic has been reported to increase the risk of persistent
patent ductus arteriosus, possibly through a prostaglandin-E-mediated process. The use of torsemide
in such patients has not been studied.
Geriatric Use
Of the total number of patients who received torsemide in United States clinical studies, 24% were 65
or older while about 4% were 75 or older. No specific age· related differences in effectiveness or safety
were observed between younger patients and elderly patients.
ADVERSE REACTIONS
At the time of approval, torsemide had been evaluated for safety in approximately 4000 subjects: over
800 of these subjects received torsemide for at least 6 months, and over 380 were treated for more
than 1 year. Among these subjects were 564 who received torsemide during United States-based trials
in which 274 other subjects received placebo.
The reported side effects of torsemide were generally transient, and there was no relationship between
side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects
occurred in 3.5% of United States patients treated with torsemide and in 4.4% of patients treated with
placebo. In studies conducted in the United States and Europe, discontinuation rates due to side effects
were 3% (38/1250) with torsemide and 3.4% (13/380) with furosemide in patients with congestive heart
failure, 2% (81409) with torsemide and 4.8% (11/230) with furosemide in patients with renal insufficiency,
and 7.6% (13/170) with torsemide and 0% (0/33) with furosemide in patients with cirrhosis.
The most common reasons for discontinuation of therapy with torsemide were (in descending order
of frequency) dizziness, headache, nausea, weakness, vomiting, hyperglycemia, excessive urination,
hyperuricemia, hypokalemia, excessive thirst, hypovolemia, impotence, esophageal hemorrhage, and
dyspepsia. Dropout rates for these adverse events ranged from 0.1 % to 0.5%.
The side effects considered possibly or probably related to study drug that occurred in United States
placebo-controlled trials in more than 1% of patients treated with torsemide are shown in Table 1.
Table 1 Reactions Possibly or Probably Drug-Related United States Placebo-Controlled
Table 1 Reactions Possibly or Probably Drug-Related United States Placebo-Controlled
Studies Incidence (Percentages of Patients)
I Torsemiae I PlaceDo
(N=564) (N=274)
Headache 7.3 9.1
Excessive Urination 6.7 2.2
Dizziness 3.2 4.0
Rhinitis 2.8 2.2
Asthenia 2.0 1.5
Diarrhea 2.0 1.1
ECG Abnormality 2.0 0.4
Cough Increase 2.0 1.5
Constipation 1.8 0.7
Nausea 1.8 0.4
Arthralgia 1.8 0.7
Dyspepsia 1.6 0.7
Sore Throat 1.6 0.7
Myalgia 1.6 1.5
Chest Pain 1.2 0.4
Insomnia 1.2 1.8
Edema 1.1 1.1
Nervousness 1.1 0.4
The daily doses of torsemide used in these trials ranged from 1.25 mg to 20 mg, with most patients
receiving 5 mg to 10 mg; the duration of treatment ranged from 1 to 52 days, with a median of 41 days.
Of the side effects listed in the table, only "excessive urination" occurred significantly more frequently
in patients treated with torsemide than in patients treated with placebo. In the placebo-controlled
hypertension studies whose design allowed side·effect rates to be attributed to dose, excessive
urination was reported by 1% of patients receiving placebo, 4% of those treated with 5 mg of daily
torsemide, and 15% of those treated with 10 mg. The complaint of excessive urination was generally
not reported as an adverse event among patients who received torsemide for cardiac, renal, or hepatic
failure.
Serious adverse events reported in the clinical studies for which a drug relationship could not be
excluded were atrial fibrillation , chest pain, diarrhea, digitalis intoxication, gastrointestinal hemorrhage,
hyperglycemia, hyperuricemia, hypokalemia, hypotension, hypovolemia, shunt thrombosis, rash, rectal
bleeding, syncope, and ventricular tachycardia.
Angioedema has been reported in a patient exposed to torsemide who was later found to be allergic
to sulfa drugs.
Of the adverse reactions during placebo-controlled trials listed without taking into account assessment
of relatedness to drug therapy, arthritis and various other nonspecific musculoskeletal problems were
more frequently reported in association with torsemide than with placebo, even though gout was
somewhat more frequently associated with placebo. These reactions did not increase in frequency or
severity with the dose of torsemide. One patient in the group treated with torsemide withdrew due to
myalgia, and one in the placebo group withdrew due to gout.
Hypokalemia
See WARNINGS.
DOSAGE AND ADMINISTRATION
General: Special dosage adjustment in the elderly is not necessary.
Because of the high bioavailability of torsemide, oral and intravenous doses are therapeutically
equivalent, so patients may be switched to and from the intravenous form with no change in dose.
Torsemide injection should be administered either slowly as a bolus over a period of 2 minutes or
administered as a continuous infusion.
If torsemide is administered through an IV line, it is recommended that, as with other IV injections,
the IV line be flushed with Normal Saline (Sodium Chloride Injection) before and after administration.
Torsemide injection is formulated above pH 8.3. Flushing the line is recommended to avoid the potential
for incompatibilities caused by differences in pH which could be indicated by color change, haziness or
the formation of a precipitate in the solution.
If torsemide injection is administered as a continuous infusion, stability has been demonstrated through
24 hours at room temperature in plastic containers for the following fluids and concentrations:
200 mg torsemide (10 mg/mL) added to:
250 mL Dextrose 5% in water
250 mL 0.9% Sodium Chloride
500 mL 0.45% Sodium Chloride
50 mg torsemide (10 mg/mL) added to:
500 mL Dextrose 5% in water
500 mL 0.9% Sodium Chloride
500 mL 0.45% Sodium Chloride
Before administration, the solution of torsemide injection should be visually inspected for discoloration
and particulate matter. If either is found, the vial should not be used.
Congestive Heart Failure
The usual initial dose is 10 mg or 20 mg of intravenous torsemide. II the diuretic response is inadequate,
the dose should be titrated upward by approximately doubling until the desired diuretic response is
obtained. Single doses higher than 200 mg have not been adequately studied.
Chronic Renal Failure
The usual initial dose of torsemide is 20 mg of intravenous torsemide. If the diuretic response is
inadequate, the dose should be titrated upward by approximately doubling until the desired diuretic
response is obtained. Single doses higher than 200 mg have not been adequately studied.
Hepatic Cirrhosis
The usual initial dose is 5 mg or 10 mg of intravenous torsemide, administered together with an
aldosterone antagonist or a potassium·sparing diuretic. If the diuretic response is inadequate, the dose
should be titrated upward by approximately doubling until the desired diuretic response is obtained.
Single doses higher than 40 mg have not been adequately studied.
Chronic use of any diuretic in hepatic disease has not been studied in adequate and well-controlled
trials.
HypertenSion
The usual initial dose is 5 mg daily. If the 5 mg dose does not provide adequate reduction in blood
pressure within 4 to 6 weeks, the dose may be increased to 10 mg daily. If the response to 10 mg is
insufficient, an additional anti-hypertensive agent should be added to the treatment regimen.
| TORSEMIDE
torsemide injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - General Injectables and Vaccines, Inc. (108250663) |
