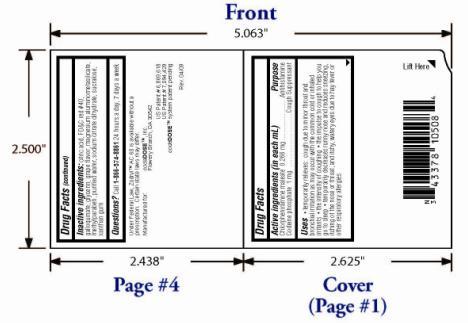
Label: ZODRYL AC 60- chlorpheniramine maleate and codeine phosphate suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 43378-105-08 - Packager: CodaDose, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: CV
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 24, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
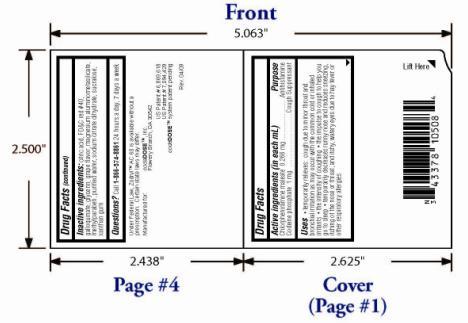
- OTC - ACTIVE INGREDIENT
-
OTC - PURPOSE
Temporarily relieves: cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants; the intensity of coughing; the impulse to cough to help you go to sleep; temporarily decreases runny nose and reduces sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever or other upper respiratory allergies
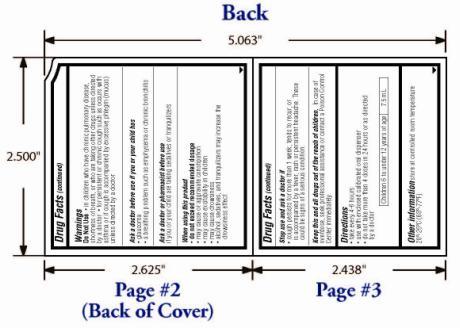
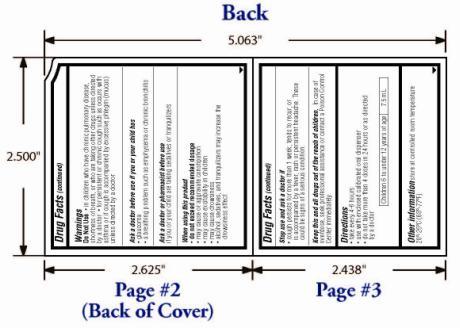
Warnings
- OTC - DO NOT USE
- OTC - ASK DOCTOR
- OTC - ASK DOCTOR/PHARMACIST
- OTC - WHEN USING
- OTC - STOP USE
-
OTC - KEEP OUT OF REACH OF CHILDREN
In case of overdose, seek professional assistance for contact a Poison Control Center immediately.
Directions:
-
Take every 4-6 hours
-
Use only with enclosed calibrated oral dispenser
-
Do not take more than 4 doses in 24 hours or as directed by a doctor
Children 6 to under 12 years of age: 7.5mL
Other information store at controlled room temperature 20°-25°C (68°-77°F).
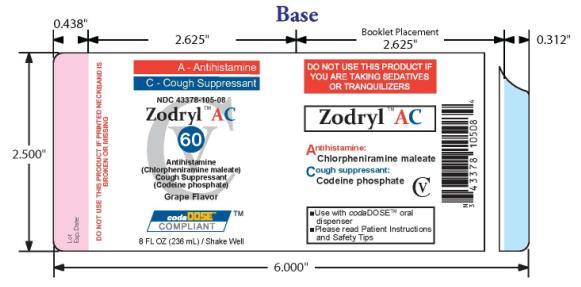
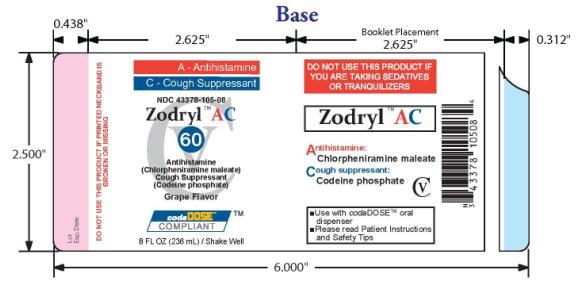
INACTIVE INGREDIENT
Citric acid, FD&C red #40, galloquinate, glycerin, grape flavor, magnesium aluminometasilicate, methylparaben, purified water, sodium citrate dihydrate, sucralose, xanthan gum
-
- OTC - QUESTIONS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZODRYL AC 60
chlorpheniramine maleate and codeine phosphate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43378-105 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 1.995 mg in 7.5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE - UNII:Q830PW7520) CODEINE PHOSPHATE 7.5 mg in 7.5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) TANNIC ACID (UNII: 28F9E0DJY6) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color red Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43378-105-08 236 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/24/2009 Labeler - CodaDose, Inc. (831355115) Registrant - Gorbec Pharmaceutical Services Inc. (791919678) Establishment Name Address ID/FEI Business Operations Gorbec Pharmaceutical Services Inc. 791919678 manufacture