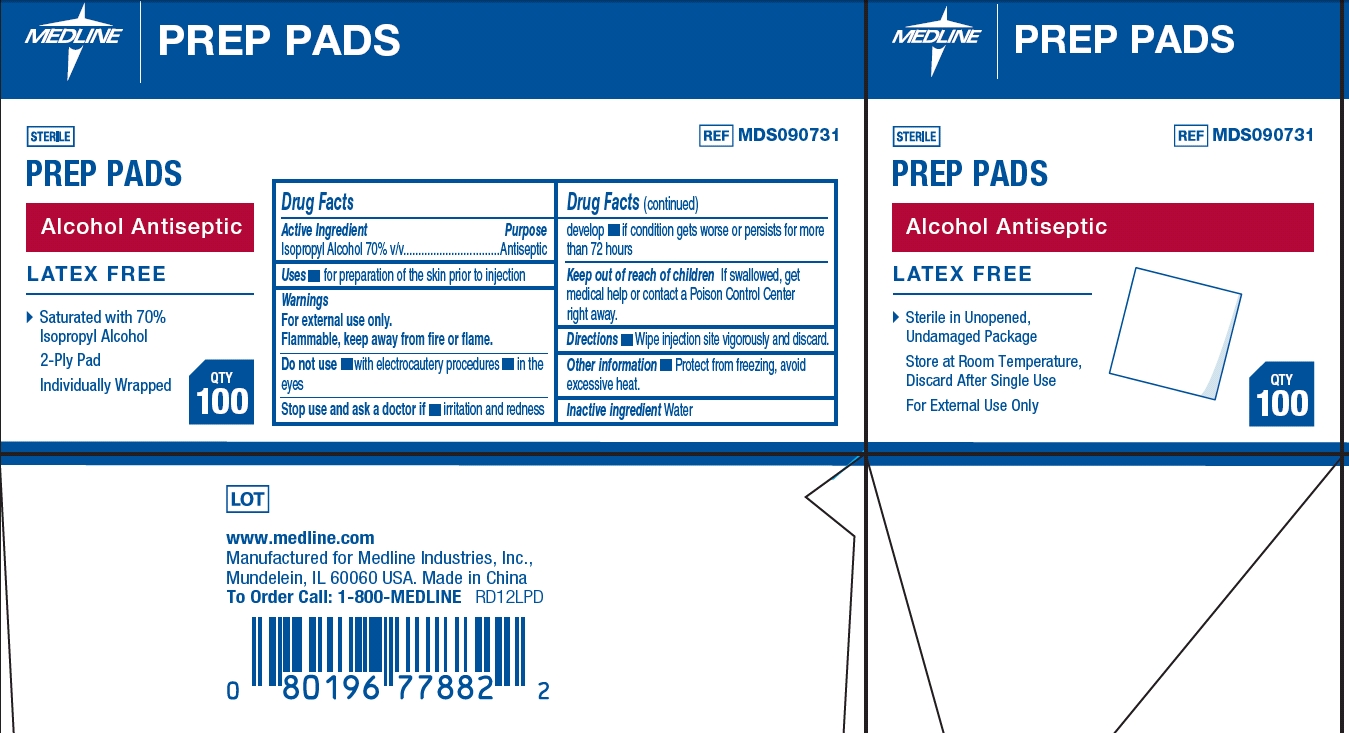
MEDLINE ALCOHOL PREP MEDIUM- isopropyl alcohol swab
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
809 Medline Prep Pads
Warnings
For external use only. Flammable, keep away from fire or flame
| MEDLINE ALCOHOL PREP
MEDIUM
isopropyl alcohol swab |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shandong Haiyan Medical Manufacture Co., Ltd | 421283439 | manufacture(53329-809) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Phoenix Innovative Healthcare Manufacturers Private Limited | 650687176 | manufacture(53329-809) | |
Revised: 12/2019
Document Id: 9a9deba5-6e56-4fc2-e053-2995a90a3727
Set id: f6f2228e-1294-45f4-942f-8ddf111e2d4e
Version: 6
Effective Time: 20191226
Medline Industries, Inc.