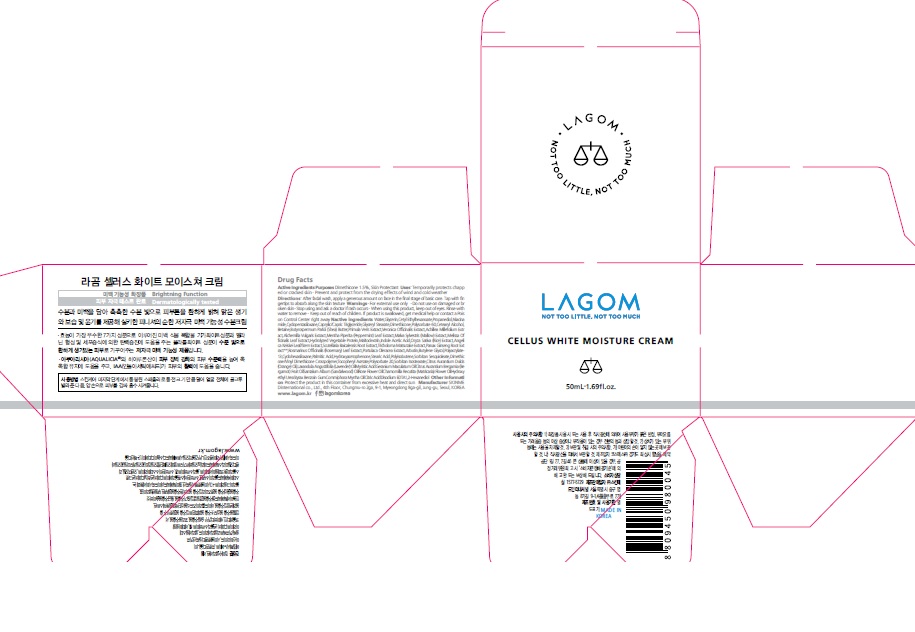
LAGOM CELLUS WHITE MOISTURE CREAM- dimethicone cream
SKINMED International Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
70738-002_Deactivation
Temporarily protects chapped or cracked skin
Prevent and protect from the drying effects of wind and cold weather
After facial wash, apply a generous amount on face in the final stage of basic care. Tap with fingertips to absorb along the skin texture
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop using and ask a doctor if rash occurs.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
Water, Glycerin,Cetyl Ethylhexanoate,Propanediol,Niacinamide,Cyclopentasiloxane,Caprylic/Capric Triglyceride,Polysorbate 60,Dimethicone,Glyceryl Stearate,Betaine,Cetearyl Alcohol,1,2-Hexanediol,Butyrospermum Parkii (Shea) Butter,Sorbitan Sesquioleate,Dimethicone/Vinyl Dimethicone Crosspolymer,Palmitic Acid,Cyclohexasiloxane,Hydroxyacetophenone,Stearic Acid,Polyacrylate-13,Tocopheryl Acetate,Polyisobutene,Yeast Amino Acids,Urea,Trehalose,Inositol,Taurine,Polysorbate 20,Citrus Aurantium Dulcis (Orange) Oil,Sorbitan Isostearate,Disodium EDTA,Phenoxyethanol,Lavandula Angustifolia (Lavender) Oil,Potassium sorbate,Geranium Maculatum Oil,Citrus Aurantium Bergamia (Bergamot) Fruit Oil,Santalum Album (Sandalwood) Oil,Rose Flower Oil,Chamomilla Recutita (Matricaria) Flower Oil,Styrax Benzoin Gum,Commiphora Myrrha Oil,Citric Acid,Alchemilla Vulgaris Extract,Primula Veris Extract,Veronica Officinalis Extract,Achillea Millefolium Extract,Mentha Piperita (Peppermint) Leaf Extract,Melissa Officinalis Leaf Extract,Malva Sylvestris (Mallow) Extract
| LAGOM CELLUS WHITE MOISTURE CREAM
dimethicone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SKINMED International Co., Ltd. (689846920) |
| Registrant - SKINMED International Co., Ltd. (689846920) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmax, Inc. | 689049693 | manufacture(70738-002) | |