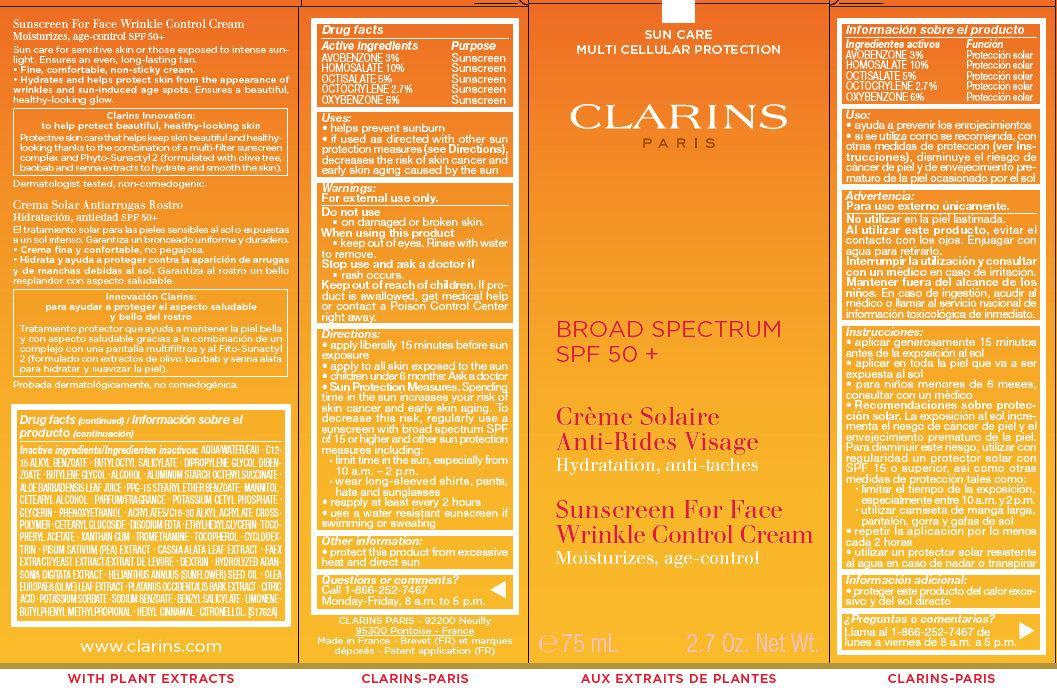
CLARINS BROAD SPECTRUM SPF 50 PLUS - SUNSCREEN FOR FACE WRINKLE CONTROL- avobenzone, homosalate, octisalate, octocrylene, oxybenzone cream
Laboratoires Clarins S.A.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CLARINS BROAD SPECTRUM SPF 50+ - SUNSCREEN FOR FACE WRINKLE CONTROL CREAM
Uses:
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions:
- apply liberally 15 minutes before sun exposure
- apply to all skin exposed to the sun
- children under 6 months: Ask a doctor
- Sun Protection Measures. Spending time in the sun in creases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
-
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- reapply at least every 2 hours
use a water resistant sun screen if swimming or sweating
Inactive ingredients
- AQUA/WATER/EAU . C12-15 ALKYL BENZOATE . BUTYLOCTYL SALICYLATE . DIPROPYLENE GLYCOL DIBENZOATE . BUTYLENE GLYCOL . ALCOHOL . ALUMINUM STARCH OCTENYLSUCCINATE . ALOE BARBADENSIS LEAF JUICE . PPG-15 STEARYL ETHER BENZOATE . MANNITOL . CETEARYL ALCOHOL . PARFUM/FRAGRANCE . POTASSIUM CETYL PHOSPHATE . GLYCERIN . PHENOXY ETHANOL . ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER . CETEARYL GLUCOSIDE . DISODIUM EDTA . ETHYLHEXYLGLYCERIN .TOCOPHERYL ACETATE . XANTHAN GUM . TROMETHAMINE . TOCOPHEROL . CYCLODEXTRIN . PISUM SATIVUM (PEA) EXTRACT . CASSIA ALATA LEAF EXTRACT . FAEX EXTRACT/YEAST EXTRACT/EXTRAIT DE LEVURE . DEXTRIN . HYDROLYZED ADANSONIA DIGITATA EXTRACT . HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL . OLEA EUROPAEA (OLIVE) LEAF EXTRACT . PLATANUS OCCIDENTALIS BARK EXTRACT . CITRIC ACID . POTASSIUM SORBATE . SODIUM BENZOATE . BENZYL SALICYLATE . LIMONENE . BUTYLPHENYL METHYLPROPIONAL . HEXYL CINNAMAL . CITRONELLOL. [S1762A]
| CLARINS BROAD SPECTRUM SPF 50 PLUS - SUNSCREEN FOR FACE WRINKLE CONTROL
avobenzone, homosalate, octisalate, octocrylene, oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Laboratoires Clarins S.A. (266317555) |
| Registrant - Laboratoires Clarins S.A. (266317555) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Laboratoires Clarins S.A. | 266317555 | manufacture(58668-4031) | |
Revised: 11/2021
Document Id: d053be5a-3e1c-6e71-e053-2995a90ae327
Set id: f5252ac5-3c22-4c6f-a6d0-caf3282fc33f
Version: 4
Effective Time: 20211108
Laboratoires Clarins S.A.