LIPIODOL ULTRA-FLUIDE- ethiodized oil injection
Delpharm Tours
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Lipiodol Ultra-Fluide
ethyl esters of iodized fatty acids of poppy seed oil
Dear Healthcare Professional,
Due to recent manufacturing issues we would like to inform you of a critical shortage of LIPIODOL ® (ETHIODIZED OIL) INJECTION . Guerbet is coordinating with the FDA to increase the availability of the LIPIODOL ® (ETHIODIZED OIL) INJECTION for US patients.
During this interim period, Guerbet, in conjunction with the FDA, is initiating a temporary importation of LIPIODOL ® ULTRA-FLUIDE , ethyl esters of iodized fatty acids of poppy seed oil, to the United States market. LIPIODOL ® ULTRA-FLUIDE contains the same drug components as LIPIODOL ® (ETHIODIZED OIL) INJECTION (manufactured by Jubliant HollisterStier, Canada). LIPIODOL ® ULTRA-FLUIDE is manufactured in compliance with European Good Manufacturing Practice (GMP) regulations by Delpharm Tours (France) for Guerbet. Delpharm Tours’s manufacturing facility is FDA inspected. The FDA has not approved this product in the United States.
At this time, no other entity except Guerbet is authorized by the FDA to import or distribute LIPIODOL ® ULTRA-FLUIDE . Any sales of LIPIODOL ® ULTRAFLUIDE ampoules from any entity other than Guerbet will be considered in violation of the Federal Food, Drug and Cosmetic Act and may be subject to enforcement action by the FDA.
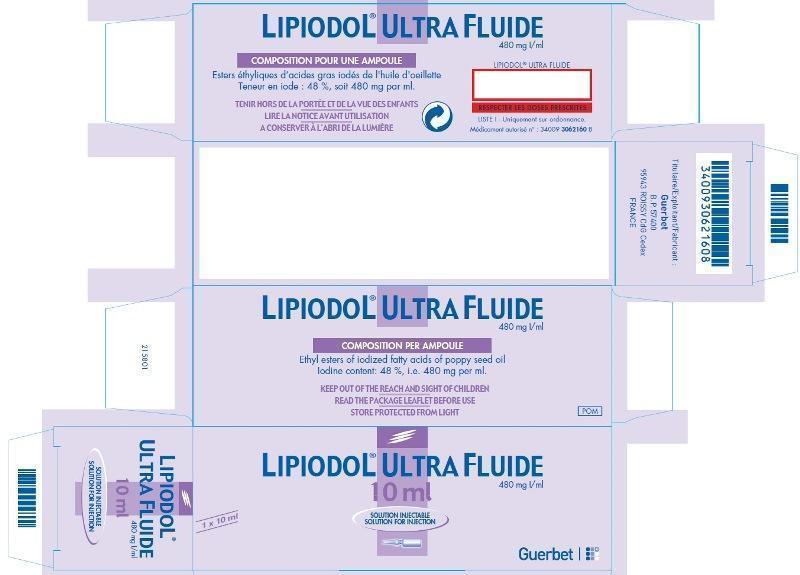
Effective immediately, Guerbet will offer the following version:
| LIPIODOL
® ULTRA-FLUIDE
48% Iodine w/vol (i.e 480 mg Iodine/mL) (ethyl esters of iodized fatty acids of poppy seed oil) |
|
| 10mL glass ampoule | Authorization# 3400930621608
Box of 1 ampoule |
LIPIODOL ® ULTRA-FLUIDE formulation is the same as LIPIODOL (Ethiodized Oil) Injection ®.
The active substance of LIPIODOL ® ULTRA-FLUIDE and LIPIODOL (ETHIODIZED OIL) INJECTION is the same (ethyl esters of iodized fatty acids of poppy seed oil, stabilized with 1% of poppy seed oil).
The barcode used on LIPIODOL ® ULTRA-FLUIDE is an international pharmaceutical manufacturing code and will likely not be recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
For questions regarding LIPIODOL ® ULTRA-FLUIDE in the United States, please contact Guerbet LLC at 1-877-729-6679 between the hours of 8 a.m. and 7 p.m. (ET), or email at info-us@guerbet-group.com.
The product comparison table below also highlights the differences between LIPIODOL ® ULTRA-FLUIDE and LIPIODOL ® (ETHIODIZED OIL) INJECTION .
Please click here for package inserts: Guerbet LIPIODOL ® ULTRA-FLUIDE ( Patient Information Leaflet and/or Summary of Product Characteristics) and LIPIODOL ® (ETHIODIZED OIL) INJECTION .
- Customers can order directly from Guerbet LLC by contacting Customer Service at 1-877-729-6679, between the hours of 8 a.m. and 7 p.m. (ET).
- LIPIODOL ® ULTRA-FLUIDE is not refundable and not for resale.
Guerbet will make reasonable attempts to fill your orders. Guerbet will be closely monitoring the distribution of LIPIODOL ® ULTRA-FLUIDE to help manage the supply.
If you have additional questions, please contact Customer Service at 1-877-729-6679, Monday through Friday, between the hours of 8 a.m. and 7 p.m. (ET), or email customer.service-us@guerbet-group.com. This communication and updated product information is available on the Guerbet website at http://www.guerbet-us.com as well as on the FDA Drug Shortage website at http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.
To report adverse events among patients administered, please call 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (ET), or email medical.liaison@guerbetgroup.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program online, by regular mail or by fax.
- Online: www.fda.gov/medwatch/report.htm
- Regular Mail: use postage-paid, pre-addressed Form FDA 3500 available at: www.fda.gov/MedWatch/getforms.htm. Mail to address on the pre-addressed form.
- Fax: 1-800-FDA-0178
We urge you to contact our Medical Information Department at 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (ET), or email medical.liaison@guerbetgroup.com if you have any questions about the information contained in this letter or the safe and effective use of LIPIODOL ® ULTRA-FLUIDE .
Comparison Table
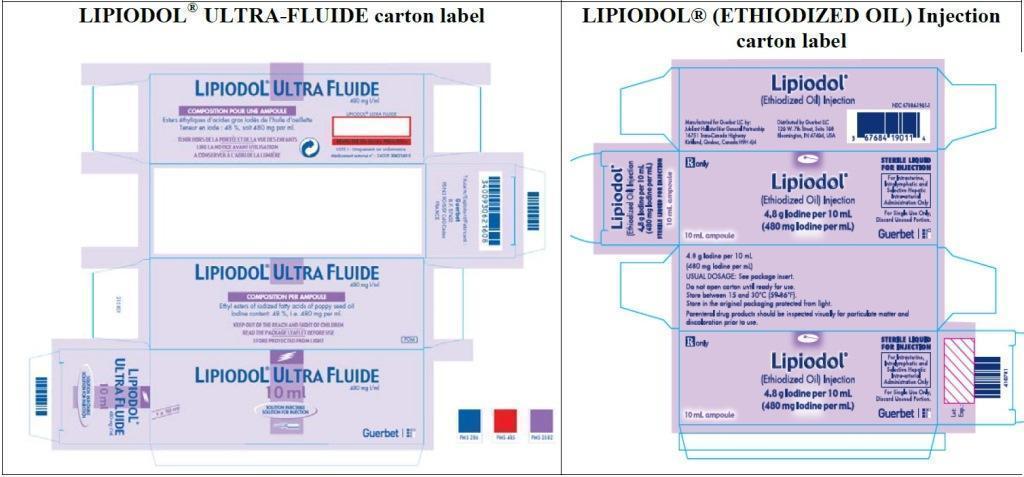
| LIPIODOL
® ULTRA-FLUIDE
(ethyl esters of iodized fatty acids of poppy seed oil) | LIPIODOL ® (ETHIODIZED OIL) INJECTION (ethyl esters of iodized fatty acids of poppy seed oil) |
| Indications and contraindications | |
| See Summary of Product Characteristics (SmPC)
Please note: see SmPC sections 4.1 Therapeutic indications, 4.2 Posology and method of administration, 4.3 Contraindications, and 4.4 Special warning and precautions for use. | LIPIODOL
® is an oil-based radio-opaque contrast
agent indicated for:
Hysterosalpingography Lipiodol hysterosalpingography is contraindicated in pregnancy, acute pelvic inflammatory disease, marked cervical erosion, endocervicitis and intrauterine bleeding, in the immediate pre-or postmenstrual phase, or within 30 days of curettage or conization. Lymphography Lipiodol Lymphography is contraindicated in patients with a right to left cardiac shunt, advanced pulmonary disease, tissue trauma or hemorrhage advanced neoplastic disease with expected lymphatic obstruction, previous surgery interrupting the lymphatic system, radiation therapy to the examined area. Selective Hepatic Intra-arterial Use Patients with HCC Lipiodol use is contraindicated in areas of the liver where the bile ducts are dilated unless external biliary drainage was performed before injection. |
| Barcode | |
| Barcode use by
LIPIODOL® ULTRA-FLUIDE may
not register accurately in the United States scanning systems. Alternative procedures should be followed to assure that the correct drug product is being used and
administered to individual patients.
| A unit of use barcode is on individual ampoules. |
| How supplied | |
| Box of 1 ampoule
Authorization# 3400930621608 | Box of 1 ampoule
NDC# 67684-1901-1 |
| Additional information | |
| Contains a patient information leaflet | N/A |
SUMMARY OF PRODUCT CHARACTERISTICS
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Corresponding to an iodine content of 480 mg/mL
in the form of ethyl esters of iodized fatty acids of poppy seed oil per 1 mL
One 10 mL ampoule contains 4800 mg of iodine
One 5 mL ampoule contains 2400 mg of iodine
Viscosity at 15°C: 70 cP (centipoise)
Viscosity at 37°C: 25 cP
Relative density at 15°C: 1.280
This medicinal product does not contain any excipients.
4. CLINICAL PARTICULARS
4.1. Therapeutic indications
In diagnostic radiology
- Lymphography
- Diagnosis of liver lesions
Diagnosis of the spread of malignant lesions, whether hepatic or not, by selective hepatic arterial injection.
In interventional radiology
- Visualisation, localisation and vectorisation during Trans-Arterial Chemo-Embolisation (TACE) of hepatocellular carcinoma at intermediate stage, in adults.
- Embolization with surgical glues
In association with surgical glues during vascular embolizations.
In endocrinology
The use of Lipiodol in prevention of iodine deficiency disorders should exclusively be reserved to countries in which other methods of supplementation, particularly iodization of salt and/or drinking water, cannot be undertaken.
4.2. Posology and route of administration
LIPIODOL ULTRA-FLUID must be administered by slow injection or by catheter, using an appropriate glass syringe and a catheter (see Section 6.2).
In diagnostic radiology
- Lymphography
Administer via a catheter inserted into a lymph duct. A dye can first be injected to locate the lymph ducts.
The usual dose is 5 to 7 mL via the strict lymphatic route to enhance contrast in an extremity (depending on the height of the subject), i.e. 10 to 14 mL for bilateral lymphography of the feet. The dose must be reduced proportionally in children. In infants 1 to 2 years of age, a dose of 1 mL per extremity is sufficient.
- Diagnosis of hepatic lesions
Strict intra-arterial route.
The usual dose varies depending on the size of the lesions, ranging from 2 to 10 mL per patient. LIPIODOL ULTRA-FLUID is sometimes mixed with small quantities of water-soluble iodinated contrast agents. Imaging must be carried out 7 to 15 days after selective injection to allow LIPIODOL ULTRA-FLUID to be eliminated from the non-tumoural liver.
Paediatric population
The dose must be reduced proportionally in children.
Patients with low weight
The dose must be reduced proportionally in this population.
Elderly
The product must be administered with special care in patients over 65 years of age with underlying diseases of the cardiovascular, respiratory or nervous systems. Keeping in mind that part of the product temporarily embolises the pulmonary capillaries, the dose must be adjusted in elderly patients with cardiorespiratory failure or the examination must be cancelled.
In interventional radiology
- Trans-Arterial Chemo-Embolisation of hepatocellular carcinoma:
The administration is by selective intra-arterial catheterism of the hepatic artery. The procedure should be performed within a typical interventional radiology setting with the appropriate equipment. The dose of LIPIODOL ULTRA-FLUID depends on the extent of the lesion, but should usually not exceed a total dose of 15 mL in adults.
LIPIODOL ULTRA-FLUID can be mixed with anticancer drugs such as cisplatin, doxorubicin, epirubicin and mitomycin. Instructions and precautions for use of the anticancer drugs must be strictly followed.
Instructions for preparation of the mixture of LIPIODOL ULTRA-FLUID with an anticancer drug:
- Prepare two syringes large enough to contain the total volume of mixture. The first syringe contains the anticancer drug solution, the second syringe contains LIPIODOL ULTRA-FLUID.
- Connect the two syringes to a 3-way stopcock.
- Perform 15 to 20 back and forth movements between the two syringes to obtain a homogeneous mixture. It is recommended to start by pushing the syringe with the anticancer drug first.
- The mixture is to be prepared at the time of use and must be used promptly after preparation (within 3 hours). If necessary during the interventional radiology procedure, the mixture can be re-homogenised as described above.
- When the adequate mixture is obtained, use a 1 to 3 mL syringe to inject in the micro-catheter.
The procedure can be repeated every 4 to 8 weeks according to tumour response and patient conditions.
Paediatric population
The efficacy and safety of the use of LIPIODOL ULTRA-FLUID for Trans-Arterial Chemo-Embolisation of hepatocellular carcinoma have not been established in children.
Elderly
The product must be administered with special care in patients over 65 years of age with underlying diseases of the cardiovascular, respiratory or nervous systems.
- Embolisation with surgical glues
Exclusive selective arterial catheterization.
The dose of LIPIODOL ULTRA-FLUID per embolisation session is determined depending on the size of the lesions. The proportion of LIPIODOL ULTRA-FLUID versus the liquid embolising agent can vary from 20 to 80% but is usually a 50/50 mixture.
The injection volume must not exceed 15 mL.
In endocrinology:
Strict intramuscular route.
- Adults and children over 4 years of age: 1 mL every three years.
- Children under 4 years of age : 0.5 mL every two years without exceeding 3 mL.
In patients with thyroid nodules, the dose is 0.2 mL.
4.3. Contraindications
- Hypersensitivity to LIPIODOL ULTRA-FLUID (ethyl esters of iodised fatty acids of poppyseed oil).
- Pregnant women
- Confirmed hyperthyroidism.
- Traumatic lesions, haemorrhage or recent bleeding (risk of extravasation or embolism).
- Bronchography (the product rapidly inundates the bronchioles and alveoli).
Contraindications specific to the use in interventional radiology:
- Trans-Arterial Chemo-Embolisation
Administration in liver areas with dilated bile ducts unless drainage has been performed.
- Embolisation with surgical glues
There are no particular contraindications apart from those of embolization, particularly in patients with portal vein thrombosis.
Contraindications specific to the use in endocrinology:
- Large multinodular goiter in patients over 45 years of age, because of the high risk of hyperthyroidism,
- During breastfeeding.
4.4. Special warnings and special precautions for use
LIPIODOL ULTRA-FLUID must not be administered intravenously, intra-arterially (apart from selective catheterisation) or intrathecally.
There is a risk of hypersensitivity whatever the dose administered.
4.4.1 Warnings
4.4.1.1. Lymphography
Pulmonary embolism occurs in most patients undergoing lymphography with injection of LIPIODOL ULTRA-FLUID, as part of the product temporarily embolises the pulmonary capillaries. It is uncommon for this embolism to be manifested clinically; should this occur, the signs are immediate (though they may appear several hours or even several days after administration) and are usually transient. For this reason, doses must be adjusted or the examination cancelled in subjects with impaired respiratory function, cardiorespiratory failure or right ventricular overload, particularly if the patient is elderly. Doses must also be reduced after antineoplastic chemotherapy or radiotherapy because lymph nodes shrink significantly and retain very little contrast agent. The injection should be carried out with radiological or endoscopic guidance. Pulmonary invasion can be reduced to the minimum by confirming radiologically that the injection is strictly intralymphatic (and not intravenous) and by discontinuing the examination as soon as the contrast agent becomes visible in the thoracic duct or as soon as lymphatic obstruction is observed.
4.4.1.2. Hypersensitivity
All iodinated contrast agents may cause minor or major hypersensitivity reactions that may be life-threatening. These hypersensitivity reactions may be either allergic (described as anaphylactic reactions when serious) or non-allergic. They may be immediate (within 60 minutes) or delayed (up to 7 days). Anaphylactic reactions occur immediately and can be fatal. They are independent of the dose, can occur after even the first dose of the product, and are often unpredictable.
Emergency resuscitation equipment must be immediately available due to the risk of a major reaction.
Patients who have previously experienced a reaction during administration of LIPIODOL ULTRA-FLUID or who have a history of hypersensitivity to iodine are at higher risk for another reaction if the product is again administered.
They are thus considered to be patients at risk.
Injection of LIPIODOL ULTRA-FLUID may exacerbate symptoms of asthma. In patients whose asthma is not controlled by treatment, the decision to use LIPIODOL ULTRA-FLUID must be based on a careful consideration of the benefit-to-risk ratio.
4.4.1.3. Thyroid
Because of the free iodine content in iodinated contrast agents, they may modify thyroid function and cause hyperthyroidism in predisposed patients. Patients at risk are those with latent hyperthyroidism or thyroid autonomy. Iodism occurs more commonly with LIPIODOL ULTRA-FLUID than with water-soluble organic iodine derivatives.
Lymphography saturates the thyroid with iodine for several months and consequently thyroid function tests must be carried out before the radiological examination.
4.4.1.4. Trans-Arterial Chemo-Embolisation
Trans-Arterial Chemo-Embolisation is not recommended in patients with decompensated liver cirrhosis (Child-Pugh ≥8), advanced liver dysfunction, macroscopic invasion and/or extra-hepatic spread of the tumour.
Hepatic intra-arterial procedures can cause an irreversible liver insufficiency in patients with serious liver malfunction and/or undergoing close multiple sessions. More than 50% liver replacement with tumour, bilirubin level greater than 2 mg/dL, lactate dehydrogenase level greater than 425 mg/dL, aspartate aminotransferase level greater than 100 IU/L and decompensated cirrhosis have been described as associated with increased post-procedural mortality.
Oesophageal varices must be carefully monitored as they can rupture immediately after treatment. If a risk of rupture is demonstrated, endoscope sclerotherapy/ligature should be performed before the Trans-Arterial Chemo-Embolisation procedure.
Iodinated contrast agent induced renal insufficiency must be systematically prevented by correct rehydration before and after The procedure.
The risk of superinfection in the treated area is normally prevented by administration of antibiotics.
4.4.1.5. Embolisation with surgical glues
An early polymerisation reaction may exceptionally occur between LIPIODOL ULTRA-FLUID and certain surgical glues, or even certain batches of glue. Before using new batches of LIPIODOL ULTRA-FLUID or surgical glue, the compatibility of LIPIODOL ULTRA-FLUID and the glue must be tested in vitro.
4.4.2 Precautions for use
4.4.2.1. Hypersensitivity
Before the examination:
identify patients at risk in a detailed interview on their history.
Corticosteroids and H1 antihistamines have been proposed as premedication in patients at greatest risk for hypersensitivity reactions (patients with known hypersensitivity to a contrast agent). However, they do not prevent the occurrence of serious or fatal anaphylactic shock.
Throughout the examination, maintain:
- medical monitoring
- an indwelling intravenous catheter.
After the examination:
After contrast agent administration, the patient must be monitored for at least 30 minutes, as most serious adverse reactions occur within this time period.
The patient must be warned of the possibility of delayed reactions (for up to seven days) (see Section 4.8 - Undesirable effects).
4.4.2.2. Thyroid
Possible thyroid risk factors must be investigated to prevent metabolic disorders. If iodinated contrast agents are to be administered to patients at risk, thyroid function tests must be carried out before the examination.
4.4.2.3. Trans-Arterial Chemo-Embolisation / Embolisation
Iodinated contrast agents can induce a transient deterioration of renal function or exacerbate pre-existing renal failure. The preventive measures are as follows:
- Identify patients at risk, i.e. patients who are dehydrated or who have renal failure, diabetes, severe heart failure, monoclonal gammopathy (multiple myeloma, Waldenstrom's macroglobulinemia), a history of renal failure after administration of iodinated contrast agents, children under one year of age and elderly atheromatous subjects.
- Hydrate the patient before and after the examination.
- Avoid combinations with nephrotoxic medicines. If such a combination is necessary, laboratory monitoring of renal function must be intensified. The medicines concerned are in particular the aminoglycosides, organoplatinums, high doses of methotrexate, pentamidine, foscarnet and certain antiviral agents [aciclovir, ganciclovir, valaciclovir, adefovir, cidofovir, tenofovir], vancomycin, amphotericin B, immunosuppressors such as cyclosporine or tacrolimus, ifosfamide)
- Allow at least 48 hours between radiological examinations or interventions with iodinated contrast agent injections, or delay further examinations or interventions until renal function returns to baseline.
- Check for lactic acidosis in diabetics treated with metformin, by monitoring serum creatinine. Normal renal function: discontinue metformin before and for at least 48 hours after contrast agent administration or until renal function returns to baseline. Abnormal renal function: metformin is contraindicated. In emergencies, if the examination is required, precautions must be taken, i.e. discontinue metformin, hydrate the patient, monitor renal function and test for signs of lactic acidosis.
- Cardiovascular and/or pulmonary co-morbidities should be assessed before initiation of a Trans-Arterial Chemo-Embolisation procedure.
4.5. Interactions with other medicinal products and other forms of interaction
Interactions with other medicines
+ Metformin
In diabetic patients, intra-arterial administration LIPIODOL ULTRA-FLUID may cause lactic acidosis induced by diminished renal function. In patients undergoing embolization or a Trans-Arterial Chemo-Embolisation, metformin must be discontinued 48 hours before the procedure and resumed no earlier than two days after the procedure.
Combinations requiring caution
+ Beta-blockers, vasoactive substances, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists.
These medicinal products reduce the efficacy of cardiovascular compensation mechanisms for blood pressure disorders. The physician must be aware of this before administering LIPIODOL ULTRA-FLUID and emergency measures must be available.
+ Diuretics
As diuretics may cause dehydration, the risk of acute renal failure is increased, particularly when high doses of contrast agents are administered.
Precautions for use: rehydration before intra-arterial administration of LIPIODOL ULTRA-FLUID for embolisation.
+ Interleukin 2
Reactions to contrast agents may be increased if the patient has recently been treated with interleukin 2 (i.v.), i.e. skin eruptions or more rarely hypotension, oliguria, or renal failure.
Interference with laboratory tests
As LIPIODOL ULTRA-FLUID remains in the body for several months, thyroid laboratory tests may be falsified for as long as two years after lymphography.
4.6. Pregnancy and lactation
Pregnancy
LIPIODOL ULTRA-FLUID must not be used in pregnant women because of the transplacental transfer of iodine, over a long period of time, which interferes probably with the thyroid function of the fœtus, with a potential risk of cerebral lesions and permanent hypothyroidism.
Breastfeeding
Pharmacokinetic studies have shown significant secretion of iodine in breast milk after intramuscular administration of LIPIODOL ULTRA-FLUID. It has been demonstrated that the iodine enters the vascular system of the breastfed infant via the gastrointestinal tract and this could interfere with thyroid function. Consequently, breastfeeding should be discontinued if LIPIODOL ULTRA-FLUID must be used.
4.7. Effects on ability to drive and use machines
No studies on the effects of LIPIODOL ULTRA-FLUID on the ability to drive and use machines have been performed.
4.8. Undesirable effects
Most of the adverse reactions are dose-related and consequently the dose should be as low as possible.
Use of LIPIODOL ULTRA-FLUID causes a foreign body reaction, with the formation of macrophages and foreign-body giant cells and the occurrence of sinus catarrh, plasmacytosis and subsequently changes in lymph node connective tissue. Healthy lymph nodes tolerate the resulting decrease in transport capacity. In patients with lymph node lesions or hypoplasia, these changes may exacerbate lymph stasis.
Hypersensitivity reactions are possible. These reactions may involve one or more effects, occurring concomitantly or successively, and usually including cutaneous, respiratory and/or cardiovascular manifestations, each of which can be a warning sign of incipient shock and, in very rare instances, can even prove fatal.
In diagnostic radiology:
- Lymphography:
A large increase in temperature followed by a fever of 38 to 39°C may occur within 24 hours following the examination.
Fat micro-embolisms may occur, with or without symptoms. In very rare cases, they may resemble embolisms originating in the body, in terms of their appearance and size. They usually appear as punctiform opacities on radiographic images of the lungs. Transient increases in temperature are possible. Fat micro-embolisms usually occur following an overdose of contrast agent or excessively rapid infusion. Anatomic anomalies such as lymphovenous fistulas or a decrease in the capacity of lymph nodes to retain the contrast agent (in elderly patients or after radiotherapy or cytostatic therapy) favour their occurrence.
Patients with a right-to-left cardiac shunt and those with a massive pulmonary embolism are particularly at risk for fat micro-embolisms in the brain.
- Diagnosis of hepatic lesions
A temperature increase is often observed. Other more rare complications may occur, i.e. nausea, vomiting and diarrhoea.
In interventional radiology:
- In Trans-Arterial Chemo-Embolisation
Most of the adverse reactions are not caused by LIPIODOL ULTRA-FLUID itself but are due to anticancer drugs or the embolisation itself.
The most frequent adverse reactions of the TACE treatment are post embolisation syndrome (fever, abdominal pain, nausea, vomiting) and transitory changes in liver function tests.
- Embolisation with surgical glues
Specific adverse reactions directly related to LIPIODOL ULTRA-FLUID have not been reported.
- In endocrinology:
Hyperthyroidism (see Section 4.4).
Adverse reactions are given in the following table according to system organ class and frequency, using the following classification: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≥ 1/10 000 to < 1/1000), very rare (< 1/10 000), undetermined frequency (cannot be estimated on the basis of available data).
|
System organ class |
Frequency: adverse reactions |
|
Immune system disorders |
Undetermined frequency: hypersensitivity, anaphylactic reaction. |
|
Endocrine disorders |
Undetermined frequency: hyperthyroidism. |
|
Nervous system disorders |
Undetermined frequency: cerebral embolism. |
|
Respiratory, thoracic and mediastinal disorders |
Undetermined frequency: pulmonary embolism. |
|
Gastrointestinal disorders |
Undetermined frequency: vomiting, diarrhoea, nausea. |
|
General disorders and administration site conditions |
Undetermined frequency: fever, pain. |
|
Injury, poisoning and procedural complications |
Rare: spinal cord injury. Undetermined frequency: fat embolism. |
Adverse reactions in children
The types of adverse reactions to LIPIODOL ULTRA-FLUID are the same as those reported in adults. Their frequency cannot be estimated on the basis of available data.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national declaration system - Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) and Regional Centers of Pharmacovigilance network – Web site: www.ansm.sante.fr
4.9. Overdose
Overdose can cause respiratory, cardiac or cerebral complications, which can be fatal. The frequency of micro-embolisms may be increased after an overdose.
The total dose of LIPIODOL ULTRA-FLUID must not exceed 20 mL.
The treatment of an overdose involves immediate symptomatic treatment and maintenance of vital functions. Establishments performing examinations with contrast agents must have emergency medicines and equipment available.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
NON-WATER-SOLUBLE CONTRAST AGENTS, Code ATC: V08AD01
(V: Other)
Used in Trans-Arterial Chemo-Embolisation by selective intra-arterial hepatic injection, LIPIODOL ULTRA-FLUID allows, as an oily contrast agent, the visualisation and control of the procedure thanks to its opacifying properties. As a vehicle, it carries and elutes anticancer drugs into hepatocellular carcinoma nodules and, as a transient embolic agent, it contributes to the vascular embolisation induced during the procedure.
As a selective intra-arterial hepatic injection procedure, Trans-Arterial Chemo-Embolisation combines the effect of a loco-regional targeted anticancer drug with the effect of an ischemic necrosis induced by dual arterio-portal embolisation. LIPIODOL ULTRA-FLUID’s opacifying properties and tropism for hepatic tumours continues for several months, so post procedure imaging can be performed for an effective patient follow-up.
5.2. Pharmacokinetic properties
After intralymphatic injection
LIPIODOL ULTRA-FLUID is released into the blood, taken up by the liver and lungs where the oily droplets are degraded in the pulmonary alveoli, spleen and adipose tissue.
After being taken up by the tissues and storage organs, reabsorption of Lipiodol occurs over a period lasting from a few days to several months or years. This is continuous and regular and the presence of iodides in the urine can be detected as long as contrast material is visible on the images.
After intramuscular injection
A portion of the oil accumulates in the muscle and adjacent tissues. Another portion is deiodinated via the metabolic route, the iodine being used to compensate for the iodine losses of the thyroid.
Urinary iodine excretion is massive and occurs rapidly (within the first few hours after the injection) but continues over the following months.
Urinary iodine excretion falls to 50 µg/day in adults within 3 to 5 years.
After selective intra-arterial injection
The iodine is eliminated mainly in the urine. After selective intra-arterial injection into the hepatic artery for the diagnostic of hepatic lesions or in Trans-Arterial Chemo-Embolisation of hepatocellular carcinoma, LIPIODOL ULTRA-FLUID is significantly more concentrated in the tumour than in the healthy liver tissue.
6. PHARMACEUTICAL PARTICULARS
6.2. Incompatibilities
Plastic is not suitable for the storage of LIPIODOL ULTRA-FLUID. In the absence of any specific compatibility studies, plastic containers and syringes should not be used.
| LIPIODOL ULTRA-FLUIDE
ethiodized oil injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Delpharm Tours (267589047) |
| Registrant - Guerbet LLC (037876096) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Delpharm Tours | 267589047 | manufacture(60694-1901) | |