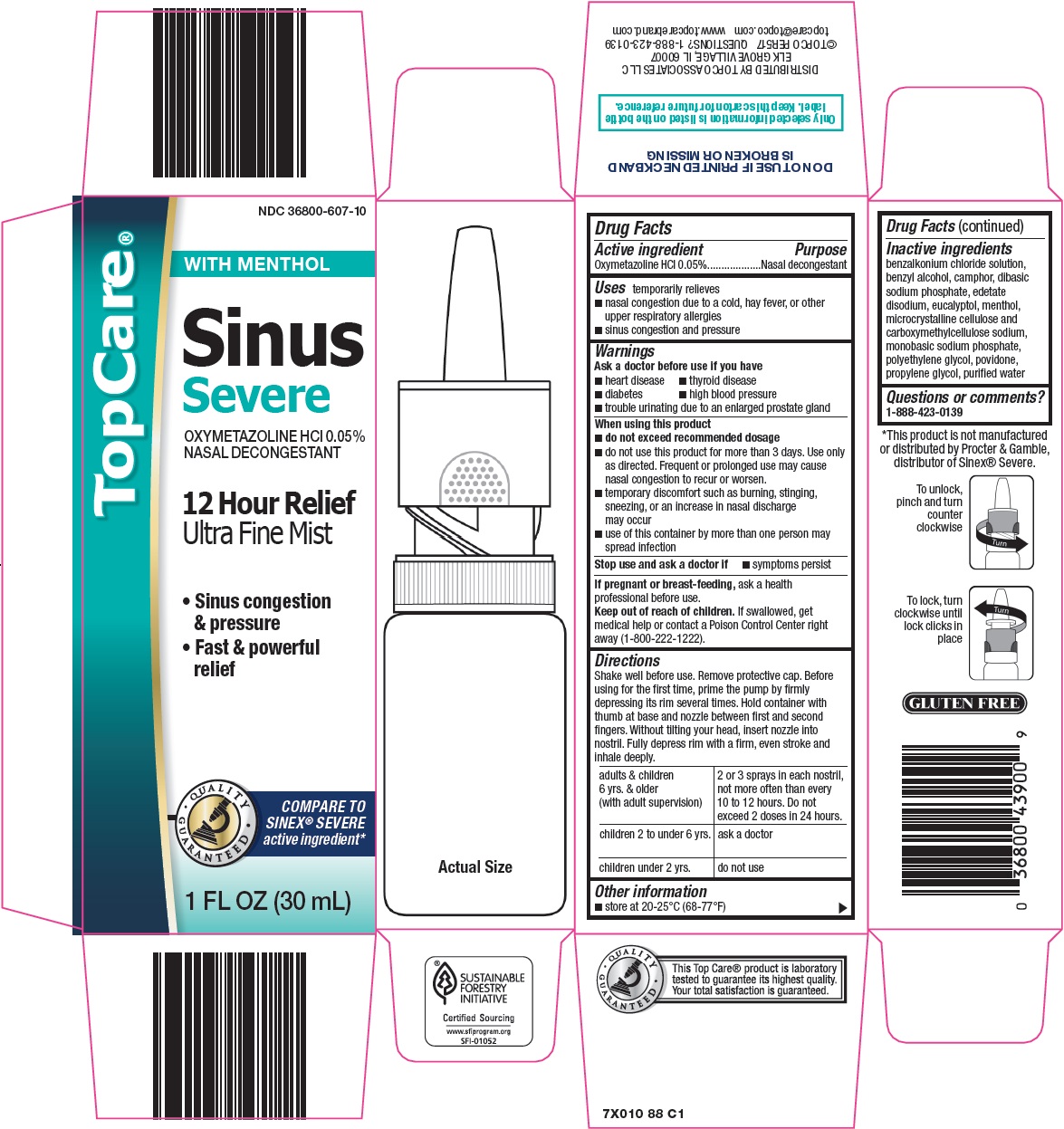
TOPCARE SINUS SEVERE- oxymetazoline hydrochloride spray
Topco Associates LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Topco Associates LLC. Sinus Severe Drug Facts
Uses
temporarily relieves
- •
- nasal congestion due to a cold, hay fever, or other upper respiratory allergies
- •
- sinus congestion and pressure
Ask a doctor before use if you have
- •
- heart disease
- •
- thyroid disease
- •
- diabetes
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not exceed recommended dosage
- •
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- •
- temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
Shake well before use. Remove protective cap. Before using for the first time, prime the pump by firmly depressing its rim several times. Hold container with thumb at base and nozzle between first and second fingers. Without tilting your head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and inhale deeply.
|
adults & children 6 yrs. & older (with adult supervision) |
2 or 3 sprays in each nostril, not more often than every 10 to 12 hours. Do not exceed 2 doses in 24 hours. |
|
children 2 to under 6 yrs. |
ask a doctor |
|
children under 2 yrs. |
do not use |
| TOPCARE SINUS SEVERE
oxymetazoline hydrochloride spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |