TARON-PREX PRENATAL- ascorbic acid, tribasic calcium phosphate, ferrous fumarate, cholecalciferol, d-alpha tocopherol, pyridoxine hydrochloride, folic acid, docosahexaenoic acid, and docusate sodium capsule
Trigen Laboratories, LLC
----------
Taron™-Prex Prenatal
with DHA CAP
SUPPLEMENT FACTS
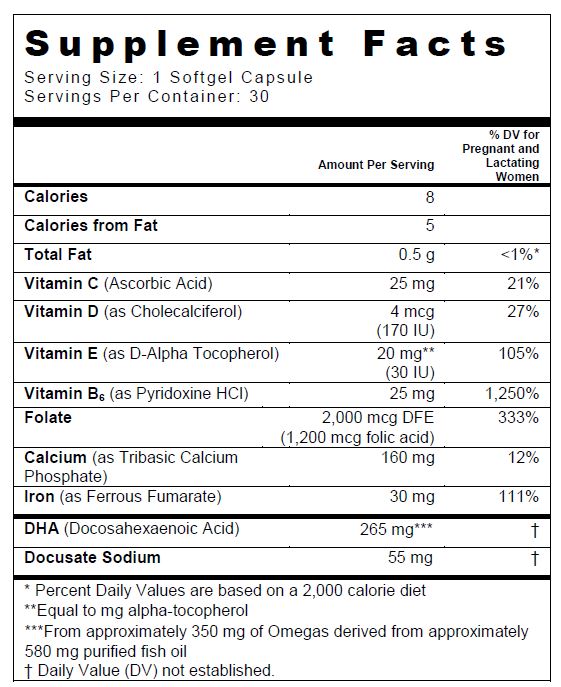
Other Ingredients: Gelatin (Bovine), Glycerin, Soy Lecithin, Purified Water, Yellow Beeswax, Natural and Artificial Orange Cream Flavor, Sorbitol, Soybean Oil, FD&C Red #40, Titanium Dioxide, Ethyl Vanillin, FD&C Yellow #6, and FD&C Blue #1
THIS PRODUCT CONTAINS SOY AND FISH OIL (ANCHOVY).
Taron™-Prex Prenatal with DHA capsules is a prescription multivitamin/mineral indicated to provide fish-based DHA supplementation throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. Taron™ Prex Prenatal with DHA may be useful in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS
Taron™-Prex Prenatal with DHA capsules are contraindicated in patients with a known hypersensitivity to any of the ingredients, including soy or fish products. Do not take this product if you are currently taking mineral oil, unless directed by a doctor.
WARNINGS
Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias here vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
DRUG INTERACTIONS
Taron™-Prex Prenatal with DHA capsules is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DESCRIPTION
Taron™-Prex Prenatal with DHA is a maroon, oblong softgel capsule imprinted with “T543”.
HOW SUPPLIED
Taron™-Prex Prenatal with DHA is dispensed in bottles of 30 softgel capsules.
Product Code: 13811-543-30
STORAGE
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°- 86°F) [See USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-877-482-3788 or FDA at 1-800-FDA-1088.
Rev. 09/2019
Customer Service: 1-877-482-3788 
Manufactured for:
Trigen Laboratories, LLC
Bridgewater, NJ 08807
www.trigenlab.com
| TARON-PREX
PRENATAL
ascorbic acid, tribasic calcium phosphate, ferrous fumarate, cholecalciferol, d-alpha tocopherol, pyridoxine hydrochloride, folic acid, docosahexaenoic acid, and docusate sodium capsule |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| flavor | ||
| imprint | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 20 mm | |
| Labeler - Trigen Laboratories, LLC (830479668) |

