DOCUSATE SODIUM- docusate sodium tablet, film coated
Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
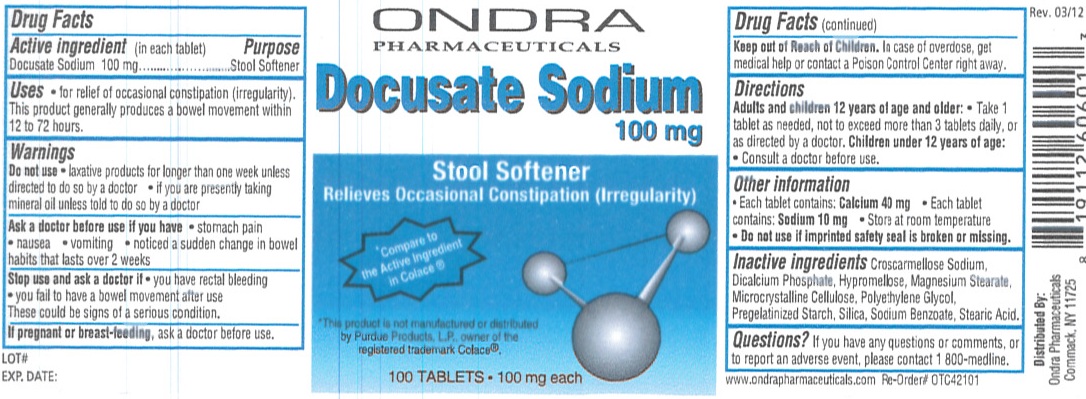
Ondra Docusate Sodium 100 mg Tablets
Uses
- for relief of occasional constipation (irregularity).This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Do not use
- laxative products for longer than one week unless directed to do so by a doctor
- if you are presently taking mineral oil unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
Adults and children 12 years of age and older: Take 1 tablet as needed, not to exceed more than 3 tablets daily, or as directed by a doctor.
Children under 12 years of age: Consult a doctor before use.
Other information
- Each tablet contains: Calcium 40 mg
- Each tablet contains: Sodium 10 mg
- Store at room temperature
- Do not use if imprinted safety seal is broken or missing
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Pregelatinized Starch, Silica, Sodium Benzoate, Stearic Acid.
Questions?
If you have any questions or comments, or to report an adverse event, please contact 1 800-medline.
Principal Display Panel
ONDRA PHARMACEUTICALS
Docusate Sodium 100 mg
Stool Softener
Relieves Occasional Constipation (Irregularity)
Compare to the Active Ingredient in Colace®
This product is not manufactured or distributed by Purdue Products, L.P.,owner of the registered trademark Colace®.
100 TABLETS 100 mg each
| DOCUSATE SODIUM
docusate sodium tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Gemini Pharmaceuticals, Inc. dba ONDRA Pharmaceuticals (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals, Inc. | 055942270 | manufacture(51645-606) | |