Label: BANMINTH 48- pyrantel tartrate powder
- NDC Code(s): 66104-2391-1
- Packager: Phibro Animal Health
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 27, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
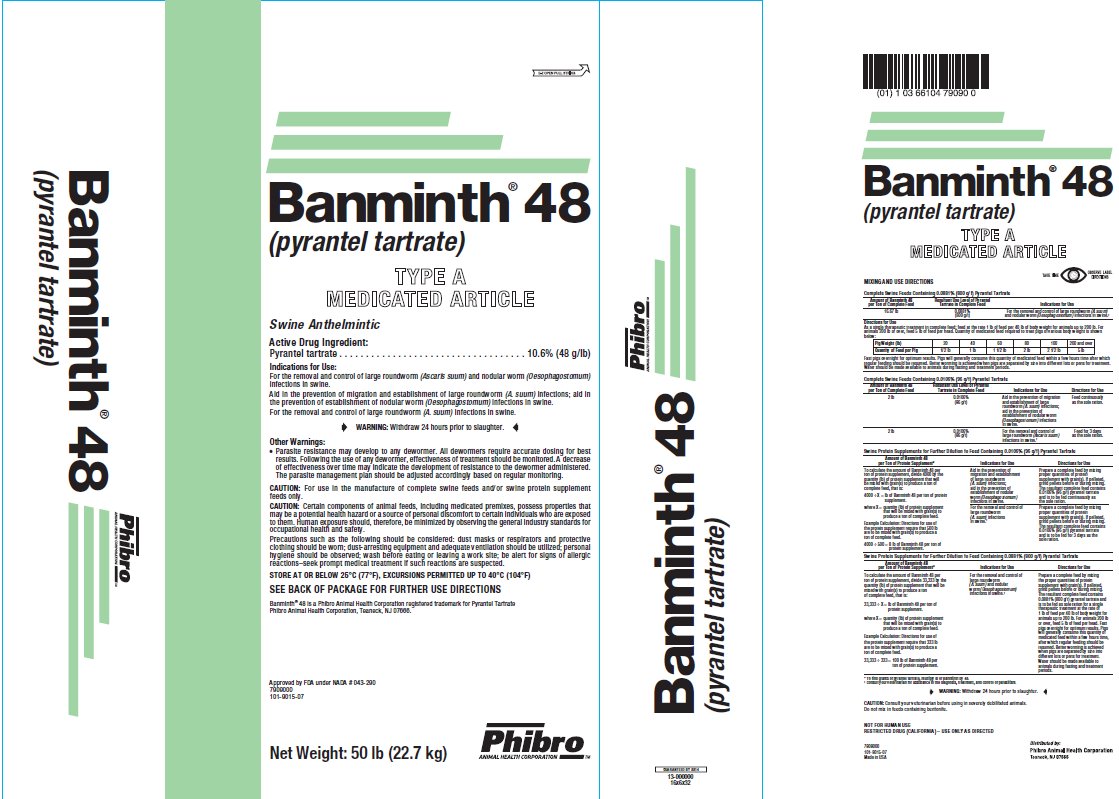
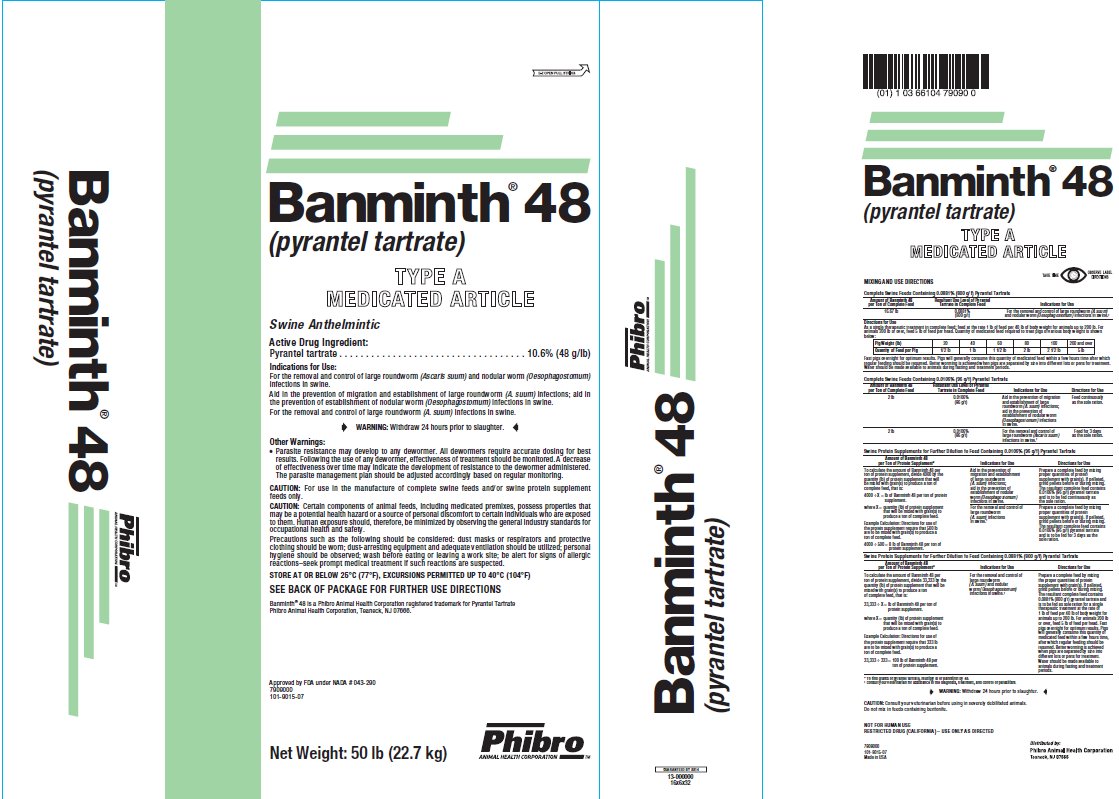
- DESCRIPTION
- Active Drug Ingredient:
-
Indications for Use:
For the removal and control of large roundworm (Ascaris suum) and nodular worm (Oesophagostomum)infections in swine.
Aid in the prevention of migration and establishment of large roundworm (A. suum) infections; aid in the prevention of establishment of nodular worm (Oesophagostomum)infections in swine.
For the removal and control of large roundworm (A. suum)infections in swine.
- WARNING:
- Other Warning:Parasite resistance may develop to any dewormer. All dewormers require accurate dosing for best results. Following the use of any dewormer, effectiveness of treatment should be monitored. A decrease of effectiveness over time may indicate the development of resistance to the dewormer administered. The parasite management plan should be adjusted accordingly based on regular monitoring. CAUTION:
-
CAUTION:
Certain components of animal feeds, including medicated premixes, possess properties that may be a potential health hazard or a source of personal discomfort to certain individuals who are exposed to them. Human exposure should, therefore, be minimized by observing the general industry standards for occupational health and safety.
Precautions such as the following should be considered: dust masks or respirators and protective clothing should be worn; dust-arresting equipment and adequate ventilation should be utilized; personal hygiene should be observed; wash before eating or leaving a work site; be alert for signs of allergic reactions–seek prompt medical treatment if such reactions are suspected.
- Store At Or Below 25°C (77°F), EXCURSIONS PERMITTED UP TO 40°C (104°F)
-
MIXING AND USE DIRECTIONS
Complete Swine Feeds Containing 0.0106% (96 g/t) Pyrantel Tartrate
Amount of Banminth 48
per Ton of Complete FeedResultant Use Level of PyranteTartrate in Complete Feed
Indications for Use
16.67 lb
0.0881%
(800g/t)For the removal and control of large roundworm (A. suum) and nodular worm (Oesophagostomum)infections in swine.†
Directions for Use
As a single therapeutic treatment in complete feed; feed at the rate 1 lb of feed per 40 lb of body weight for animals up to 200 lb. For animals 200 lb or over, feed 5 lb of feed per head. Quantity of medicated feed required to treat pigs of various body weight is shown below:
Pig Weight (lb)
20
40
60
80
100
200 and over
Quantity of Feed per Pig
1/2 lb
1 lb
1 1/2 lb
2 lb
2 1/2 lb
5 lb
Fast pigs overnight for optimum results. Pigs will generally consume this quantity of medicated feed within a few hours time after which regular feeding should be resumed. Better worming is achieved when pigs are separated by size into different lots or pens for treatment. Water should be made available to animals during fasting and treatment periods.
Complete Swine Feeds Containing 0.0106% (96 g/t) Pyrantel Tartrate
Amount of Banminth 48
per Ton of Complete FeedResultant Use Level of Pyrantel Tartrate in Complete Feed
Indications for Use
Directions for Use
2 lb
0.0106%
(96 g/t)Aid in the prevention of migration and establishment of large roundworm (A. suum) infections; aid in the prevention of establishment of nodular worm (Oesophagostomum) infections in swine.
Feed continuously as the sole ration
2 lb
0.0106%
(96 g/t)For the removal and control of large roundworm (Ascaris suum) infections in swine.†
Feed for 3 days as the sole ration.
Swine Protein Supplements for Further Dilution to Feed Containing 0.0106% (96 g/t) Pyrantel Tartrate
Amount of Banminth 48
per Ton of Protein Supplement*Indications for Use
Directions
To calculate the amount of Banminth 48 per ton of protein supplement, divide 4000 by the quantity (lb) of protein supplement that will be mixed with grain(s) to produce a ton of complete feed, that is:
4000 ÷ X = lb of Banminth 48 per ton of protein supplement.
where X = quantity (lb) of protein supplement that will be mixed with grain(s) to produce a ton of complete feed.
Example Calculation: Directions for use of the protein supplement require that 500 lb are to be mixed with grain(s) to produce a ton of complete feed.
4000 ÷ 500 = 8 lb of Banminth 48 per
ton of protein supplement.
Aid in the prevention of migration and establishment of large roundworm (A. suum)infections; aid in the prevention of establishment of nodular worm (Oesophagostomum) infections in swine.
Prepare a complete feed by mixing proper quantities of protein supplement with grain(s). If pelleted, grind pellets before or during mixing. The resultant complete feed contains 0.0106% (96 g/t) pyrantel tartrate and is to be fed continuously as the sole ration.
For the removal and control of large roundworm (A. suum) infections in swine.†
Prepare a complete feed by mixing proper quantities of protein supplement with grain(s). If pelleted, grind pellets before or during mixing. The resultant complete feed contains 0.0106% (96 g/t) pyrantel tartrate and is to be fed for 3 days as the sole ration.
Swine Protein Supplements for Further Dilution to Feed Containing 0.0881% (800 g/t) Pyrantel Tartrate
Amount of Banminth 48
per Ton of Protein Supplement*Indications for Use
Directions for Use
To calculate the amount of Banminth 48 per ton of protein supplement, divide 33,333 by the quantity (lb) of protein supplement that will be mixed with grain(s) to produce a ton of complete feed, that is:
33,333 ÷ X = lb of Banminth 48 per ton of protein supplement.
where X = quantity (lb) of protein supplement that will be mixed with grain(s) to produce a ton of complete feed.
Example Calculation: Directions for use of the protein supplement require that 333 lb are to be mixed with grain(s) to produce a ton of complete feed.
33,333 ÷ 333 = 100 lb of Banminth 48 per ton of protein supplement.
For the removal and control of large roundworm (A. suum)and nodular worm (Oesophagostomum) infections in swine.†
Prepare a complete feed by mixing the proper quantities of protein supplement with grain(s). If pelleted, grind pellets before or during mixing. The resultant complete feed contains 0.0881% (800 g/t) pyrantel tartrate and is to be fed as sole ration for a single therapeutic treatment at the rate of 1 lb of feed per 40 lb of body weight for animals up to 200 lb. For animals 200 lb or over, feed 5 lb of feed per head. Fast pigs overnight for optimum results. Pigs will generally consume this quantity of medicated feed within a few hours time, after which regular feeding should be resumed. Better worming is achieved when pigs are separated by size into different lots or pens for treatment. Water should be made available to animals during fasting and treatment periods.
* To find grams of pyrantel tartrate, multiply lb of Banminth by 48.
† Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
- WARNING:
-
SPL UNCLASSIFIED SECTION
SEE BACK OF PACKAGE FOR FURTHER USE DIRECTIONS
Banminth® 48 is a Phibro Animal Health Corporation registered trademark for Pyrantel Tartrate
Phibro Animal Health Corporation, Teaneck, NJ 07666.
Net Weight: 50 lb (22.7 kg)
Approved by FDA under NADA #043-290
7909000
101-9015-07
U.S. Patent No. 3,549,624
Made in USA
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BANMINTH 48
pyrantel tartrate powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:66104-2391 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL (UNII: 4QIH0N49E7) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 48 g in 0.45 kg Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) CALCIUM CARBONATE (UNII: H0G9379FGK) SOYBEAN (UNII: L7HT8F1ZOD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66104-2391-1 22.7 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA043290 02/06/1973 Labeler - Phibro Animal Health (006989008) Registrant - Phibro Animal Health (006989008)