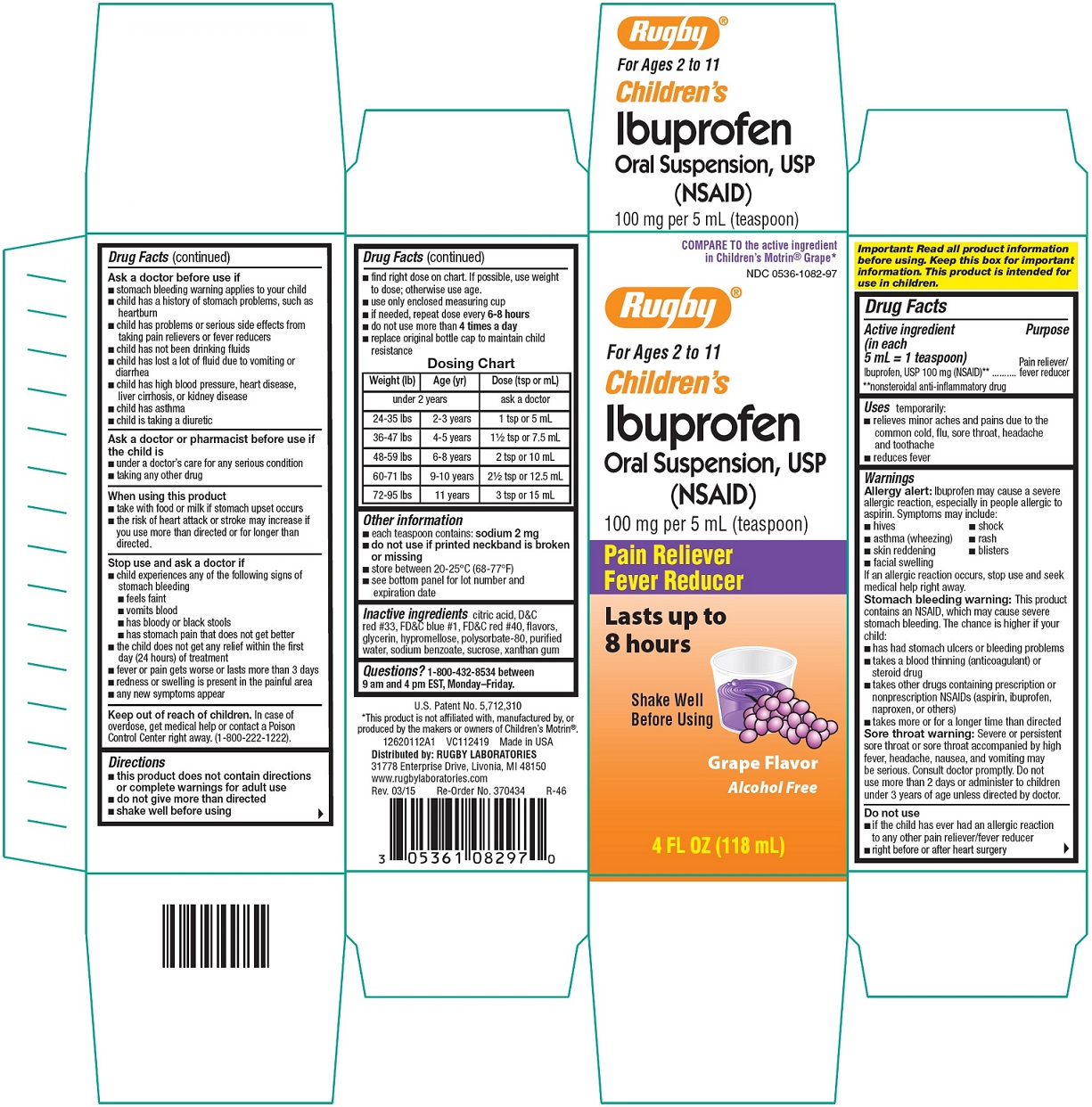
CHILDRENS IBUPROFEN- ibuprofen suspension
Rugby Laboratories
----------
Ibuprofen Oral Suspension, USP
ACTIVE INGREDIENT
(in each 5 mL = 1 teaspoon)
Ibuprofen, USP 100 mg (NSAID)**
**nonsteroidal anti-inflammatory drug
USES
temporarily:
- relieves minor aches and pains due to the common cold, flu, sore throat, headache and toothache
- reduces fever
WARNINGS
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- shock
- asthma (wheezing)
- rash
- skin reddening
- blisters
- facial swelling
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
DO NOT USE
- if the child has ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
ASK DOCTOR BEFORE USE IF
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has problems or serious side effects from taking pain relievers or fever reducers
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child has high blood pressure, heart disease, liver cirrhosis, or kidney disease
- child has asthma
- child is taking a diuretic
ASK DOCTOR OR PHARMACIST BEFORE USE IF THE CHILD IS
- under a doctor’s care for any serious condition
- taking any other drug
WHEN USING THIS PRODUCT
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
STOP USE AND ASK A DOCTOR IF
- child experiences any of the following signs of stomach bleeding
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- the child does not get any relief within the first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
DIRECTIONS
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- use only enclosed measuring cup
- if needed, repeat dose every 6-8 hours
- do not use more than 4 times a day
- replace original bottle cap to maintain child resistance
| Weight (lb) | Age (yr) | Dose (tsp or mL) |
|---|---|---|
| under 2 years | ask a doctor |
|
| 24 – 35 lbs | 2 – 3 years | 1 tsp or 5 mL |
| 36 – 47 lbs | 4 – 5 years | 1½ tsp or 7.5 mL |
| 48 – 59 lbs | 6 – 8 years | 2 tsp or 10 mL |
| 60 – 71 lbs | 9 – 10 years | 2½ tsp or 12.5 mL |
| 72 – 95 lbs | 11 years | 3 tsp or 15 mL |
Other information
- each teaspoon contains: sodium 2 mg
- do not use if printed neckband is broken or missing
- store between 20 - 25°C (68 - 77°F)
- see bottom panel for lot number and expiration date
INACTIVE INGREDIENT
citric acid, D&C red #33, FD&C blue #1, FD&C red #40, flavors, glycerin, hypromellose, polysorbate 80, purified water, sodium benzoate, sucrose, xanthan gum
QUESTIONS?
1-800-432-8534 between 9 am and 4 pm EST, Monday – Friday.
Generic Section
U.S. Patent No. 5,712,310
*This product is not affiliated with, manufactured by, or produced by the makers or owners of Children’s Motrin®
12620112A1
VC112419
Made in USA
Distributed By:
RUGBY LABORATORIES
31778 Enterprise Drive, Livonia, MI 48150
www.rugbylaboratories.com
Rev. 03/15 Re-Order No. 370434 R-46
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Compare to the active ingredient in Children’s Motrin® Grape*
NDC 0536-1082-97
For ages 2 to 11
CHILDREN’S Ibuprofen Oral Suspension, USP
(NSAID)
100 mg per 5 mL (teaspoon)
Pain Reliever
Fever Reducer
Lasts up to 8 hours
Grape Flavor
Alcohol Free
4 FL OZ (118 mL)
| CHILDRENS IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |