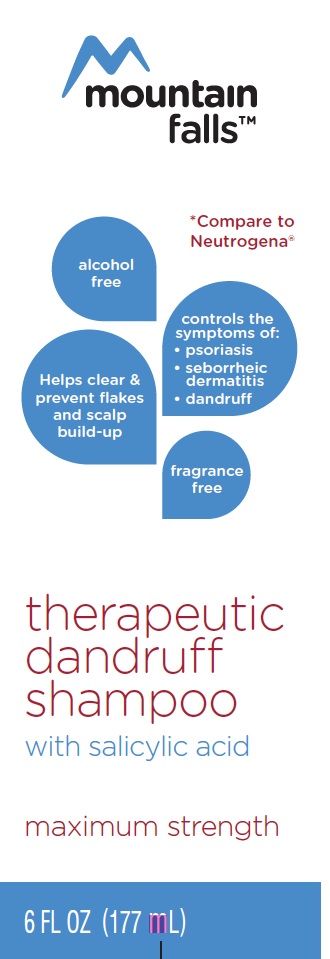
THERAPEUTIC DANDRUFF- dandruff shampoo shampoo
Consumer Product Partners, LLC
----------
Dandruff Shampoo
975
Stop use and ask a doctor if
condition worsens or does not improve after regular use of this product as directed
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- wet hair thoroughly
- massage shampoo into the scalp
- lather. Leave on hair and scalp for several minutes.
- rinse and repeat
- for best results use at least twice a week or as directed by a doctor
Inactive ingredients
water, cocamidopropyl betaine, sodium C14-16 olefin sulfonate, linoleamidopropyl PG-dimonium chloride phosphate, sodium lauroyl sarcosinate, polyquaternium-22, hexylene glycol, sodium citrate
*This product is not manufactured or distributed by Neutrogena Corporation, Distributor of Neutrogena T/Sal Therapeutic Shampoo
mountain falls therapeutic dandruff shampoo with 3% salicylic acid
Manufactured by: Vi-Jon, Inc.,
St. Louis, MO 63114
Questions or Comments? 1-888-593-0593
Made in the USA
with US and foreign parts.
| THERAPEUTIC DANDRUFF
dandruff shampoo shampoo |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Consumer Product Partners, LLC (119091520) |
| Registrant - Consumer Product Partners, LLC (119091520) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon, LLC | 088520668 | manufacture(11344-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon, LLC | 790752542 | manufacture(11344-975) | |