CEPACOL INSTAMAX SORE THROAT ARCTIC CHERRY- benzocaine and menthol, unspecified form lozenge
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cēpacol
®Instamax
®
Sore Throat Lozenges Arctic Cherry
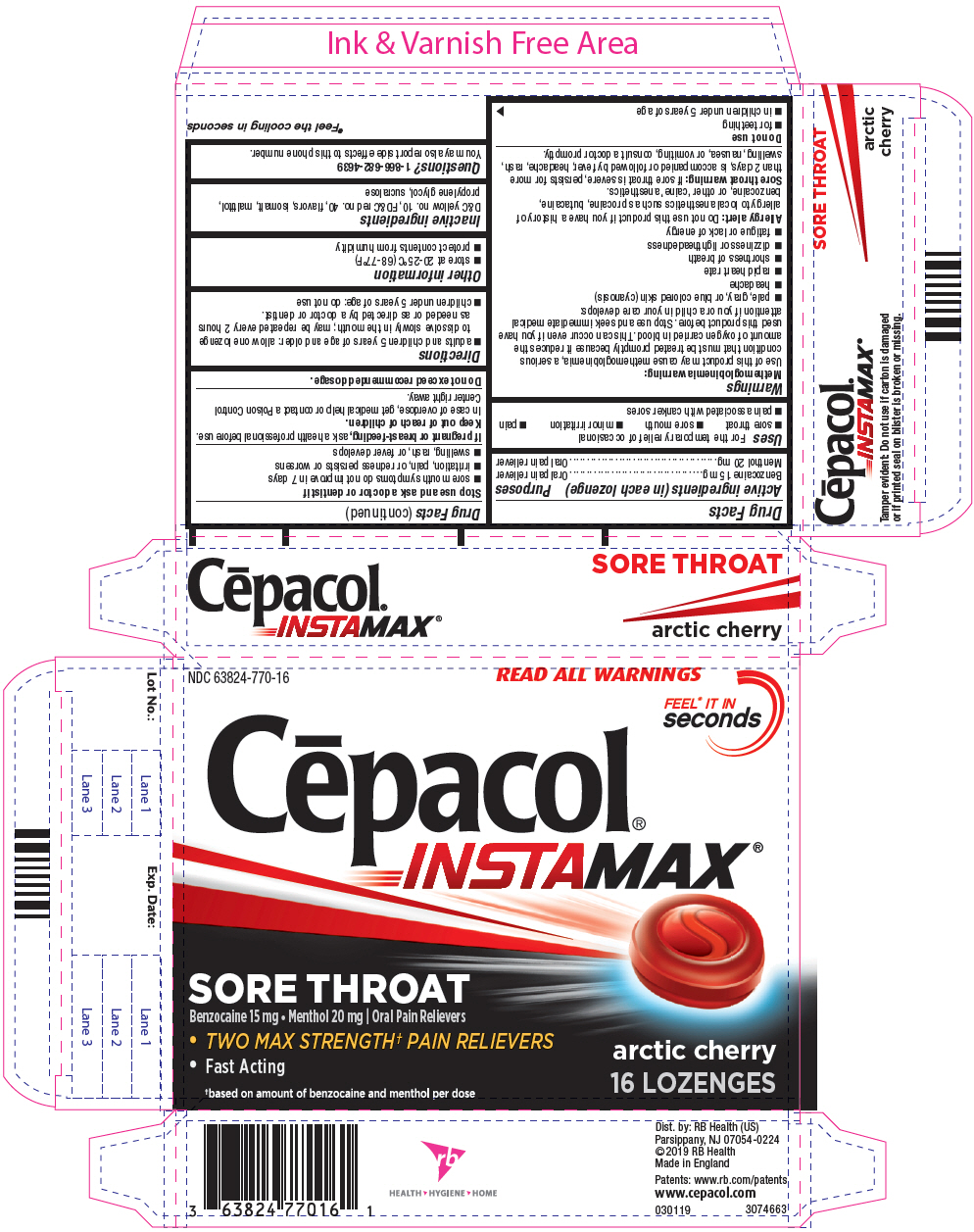
Uses
For the temporary relief of occasional
- sore throat
- sore mouth
- minor irritation
- pain
- pain associated with canker sores
Warnings
Methemoglobinemia warning
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other 'caine' anesthetics.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
Directions
- adults and children 5 years of age and older: allow one lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use
Inactive ingredients
D&C yellow no. 10, FD&C red no. 40, flavors, isomalt, maltitol, propylene glycol, sucralose
PRINCIPAL DISPLAY PANEL - 16 Lozenge Blister Pack Carton
NDC 63824-770-16
READ ALL WARNINGS
FEEL* IT IN
seconds
Cēpacol
®
INSTAMAX
®
SORE THROAT
Benzocaine 15 mg • Menthol 20 mg | Oral Pain Relievers
- TWO MAX STRENGTH †PAIN RELIEVERS
- Fast Acting
arctic cherry
16 LOZENGES
†based on amount of benzocaine and menthol per dose
| CEPACOL INSTAMAX
SORE THROAT ARCTIC CHERRY
benzocaine and menthol, unspecified form lozenge |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |