TRIVEEN-PRX
RNF- folic acid, ascorbic acid, tribasic calcium phosphate, iron, cholecalciferol, alpha-tocopherol, pyridoxine hydrochloride, doconexent, and docusate sodium capsule
Trigen Laboratories, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
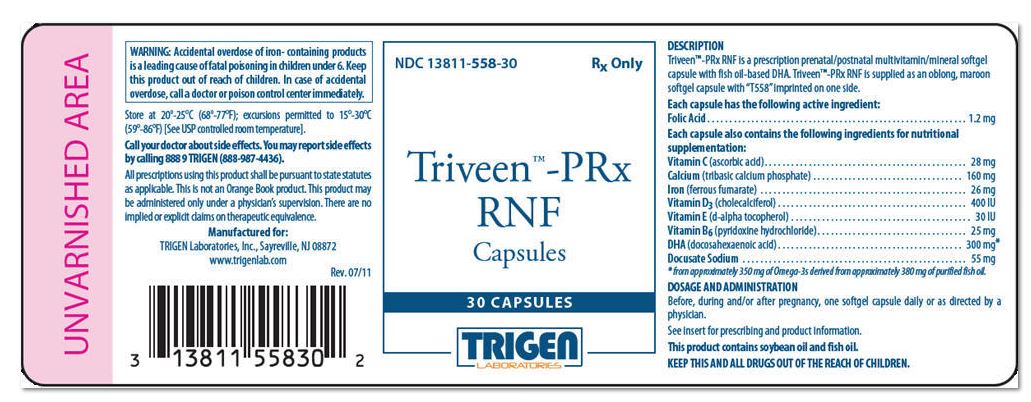
Triveen™-PRx RNF
Capsules
Rx Only
DESCRIPTION
Triveen™-PRx RNF is a prescription prenatal/postnatal multivitamin/mineral softgel capsule with fish oil-based DHA. Triveen™-PRx RNF is supplied as an oblong, maroon softgel capsule with "T558" imprinted on one side.
| Each capsule has the following active ingredient: | |
| Folic Acid | 1.2 mg |
| *from approximately 350 mg of Omega-3s derived from approximately 380 mg of purified fish oil. | |
| Each capsule also contains the following ingredients for nutritional supplementation: | |
| Vitamin C (ascorbic acid) | 28 mg |
| Calcium (tribasic calcium phosphate) | 160 mg |
| Iron (ferrous fumarate) | 26 mg |
| Vitamin D 3 (cholecalciferol) | 400 IU |
| Vitamin E (d-alpha tocopherol) | 30 IU |
| Vitamin B 6 (pyridoxine hydrochloride) | 25 mg |
| DHA (Docosahexaenoic Acid) | 300 mg * |
| Docusate Sodium | 55 mg |
Inactive Ingredients
Gelatin, Glycerin, Soybean Oil, Purified water, Lecithin, Yellow Beeswax, Natural Creamy Orange Flavor, FD&C Red #40, Titanium Dioxide, Ethyl Vanillin, FD&C Yellow #6, FD&C Blue #1.
INDICATIONS AND USAGE
Triveen™-PRx RNF is indicated for the supplemental requirements of patients with nutritional deficiencies or are in need of nutritional supplementation.
CONTRAINDICATIONS
Triveen™-PRx RNF capsules is contraindicated in patients with a known hypersensitivity to any of the ingredients, including fish or fish oil. Do not take this product if you are presently taking mineral oil, unless directed by a doctor.
WARNINGS
Daily ingestion of more than 3 g per day of omega-3 fatty acids (ALA, EPA, and DHA) from fish oils may have potential antithrombotic activities, or effects, and may increase bleeding times. Administration of omega-3 fatty acids – including DHA, should be avoided in patients with inherited or acquired bleeding diatheses, including those taking anticoagulants.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
This product contains soybean oil and fish oil.
CAUTION
Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) per day.
PRECAUTIONS
General
Folic acid, when administered as a single agent in doses above 0.1 mg daily, may obscure the detection of B
12 deficiency (specifically, the administration of folic acid may reverse the hematological manifestations of B
12 deficiency, including pernicious anemia, while not addressing the neurological manifestations). Reduced folates may be less likely than folic acid to mask vitamin B
12 deficiency. Folate therapy alone is inadequate for the treatment of B
12 deficiency.
PATIENT INFORMATION
Triveen™-PRx RNF is a prescription vitamin for use only under the direction and supervision of a licensed physician.
INTERACTIONS
Pyridoxine hydrochloride should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
Drugs which may interact with folate include:
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate. Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the level of this AED in the blood and allowing breakthrough seizures to occur.
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels. DHFRIs include aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim.
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments. NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- Pancreatic Enzymes: Reduced folate levels have occurred in some patients taking pancreatic extracts.
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid, as well as possibly the use of other forms of folates – including reduced folates. Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride.
DOSAGE AND ADMINISTRATION
Before, during and/or after pregnancy, one softgel capsule daily or as directed by a physician.
HOW SUPPLIED
Bottles of 30 softgel capsules.
NDC 13811-558-30
Bottles of 60 softgel capsules.
NDC 13811-558-60
STORAGE
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP controlled room temperature].
Call your doctor about side effects. You may report by calling 888 9 TRIGEN (888-987-4436).
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Rx Only
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
TRIGEN Laboratories, Inc.
Sayreville, NJ 08872
www.trigenlab.com
Rev. 08/11
| TRIVEEN-PRX
RNF
folic acid, ascorbic acid, tribasic calcium phosphate, iron, cholecalciferol, alpha-tocopherol, pyridoxine hydrochloride, doconexent, and docusate sodium capsule |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Trigen Laboratories, LLC (830479668) |