ISOSORBIDE MONONITRATE- isosorbide mononitrate tablet, film coated, extended release
American Health Packaging
----------
ISOSORBIDE MONONITRATE
EXTENDED-RELEASE
TABLETS, USP
DESCRIPTION
Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins.
Isosorbide Mononitrate Extended-Release Tablets, USP, for oral administration, contain 30 mg or 60 mg of isosorbide mononitrate in an extended-release formulation. In addition, each tablet contains the following inactive ingredients: ammonium phosphate dibasic, anhydrous lactose, carnauba wax, colloidal silicon dioxide, magnesium stearate, and synthetic paraffin wax. Film coating and polishing solution contains: hypromellose, polyethylene glycol, red iron oxide (30 mg tablet only), titanium dioxide, and yellow iron oxide (60 mg tablet only).
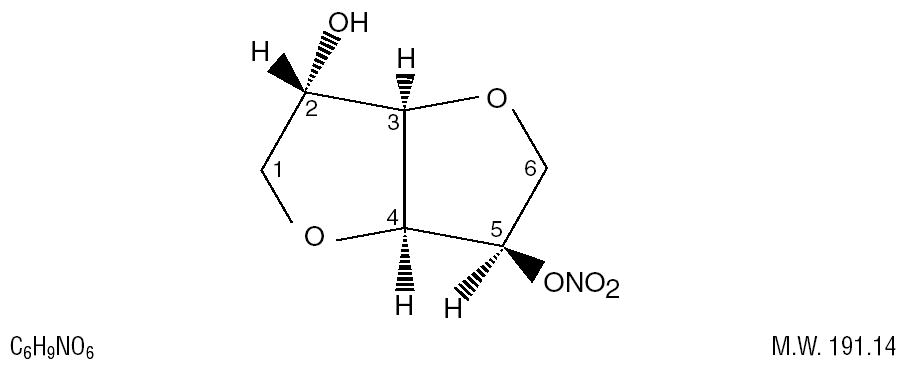
The chemical name for ISMN is 1,4:3,6-dianhydro-, D-glucitol 5-nitrate; the compound has the following structural formula:
ISMN is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of about 90°C, and an optical rotation of +144° (2% in water, 20°C).
Isosorbide mononitrate is freely soluble in water, ethanol, methanol, chloroform, ethyl acetate, and dichloromethane. This drug product meets USP Dissolution Test No. 3.
CLINICAL PHARMACOLOGY
Mechanism of Action
The Isosorbide Mononitrate product is an oral extended-release formulation of ISMN, the major active metabolite of isosorbide dinitrate; most of the clinical activity of the dinitrate is attributable to the mononitrate.
The principal pharmacological action of ISMN and all organic nitrates in general is relaxation of vascular smooth muscle, producing dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, and systolic arterial pressure and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined.
Pharmacodynamics
Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the antianginal efficacy of continuously delivered nitrates. In the large majority of these trials, active agents were indistinguishable from placebo after 24 hours (or less) of continuous therapy. Attempts to overcome tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours has their antianginal efficacy been restored. Isosorbide Mononitrate Tablets during long-term use over 42 days dosed at 120 mg once daily continued to improve exercise performance at 4 hours and at 12 hours after dosing, but its effects (although better than placebo) are less than or, at best, equal to the effects of the first dose of 60 mg.
Pharmacokinetics and Metabolism
After oral administration of ISMN as a solution or immediate-release tablets, maximum plasma concentrations of ISMN are achieved in 30 to 60 minutes, with an absolute bioavailability of approximately 100%. After intravenous administration, ISMN is distributed into total body water in about 9 minutes with a volume of distribution of approximately 0.6 to 0.7 L/kg. Isosorbide mononitrate is approximately 5% bound to human plasma proteins and is distributed into blood cells and saliva. Isosorbide mononitrate is primarily metabolized by the liver, but unlike oral isosorbide dinitrate, it is not subject to first-pass metabolism. Isosorbide mononitrate is cleared by denitration to isosorbide and glucuronidation as the mononitrate, with 96% of the administered dose excreted in the urine within 5 days and only about 1% eliminated in the feces. At least six different compounds have been detected in urine, with about 2% of the dose excreted as the unchanged drug and at least five metabolites. The metabolites are not pharmacologically active. Renal clearance accounts for only about 4% of total body clearance. The mean plasma elimination half-life of ISMN is approximately 5 hours.
The disposition of ISMN in patients with various degrees of renal insufficiency, liver cirrhosis, or cardiac dysfunction was evaluated and found to be similar to that observed in healthy subjects. The elimination half-life of ISMN was not prolonged, and there was no drug accumulation in patients with chronic renal failure after multiple oral dosing.
The pharmacokinetics and/or bioavailability of Isosorbide Mononitrate Extended-Release Tablets have been studied in both normal volunteers and patients following single- and multiple-dose administration. Data from these studies suggest that the pharmacokinetics of ISMN administered as Isosorbide Mononitrate Tablets are similar between normal healthy volunteers and patients with angina pectoris. In single- and multiple-dose studies, the pharmacokinetics of ISMN were dose proportional between 30 mg and 240 mg.
In a multiple-dose study, the effect of age on the pharmacokinetic profile of Isosorbide Mononitrate 60 mg and 120 mg (2 x 60 mg) Tablets was evaluated in subjects ≥45 years. The results of that study indicate that there are no significant differences in any of the pharmacokinetic variables of ISMN between elderly (≥65 years) and younger individuals (45-64 years) for the isosorbide mononitrate 60 mg dose. The administration of Isosorbide Mononitrate Tablets 120 mg (2 x 60 mg tablets every 24 hours for 7 days) produced a dose-proportional increase in Cmax and AUC, without changes in Tmax or the terminal half-life. The older group (65-74 years) showed 30% lower apparent oral clearance (Cl/F) following the higher dose, i.e., 120 mg, compared to the younger group (45-64 years); CI/F was not different between the two groups following the 60 mg regimen. While CI/F was independent of dose in the younger group, the older group showed slightly lower CI/F following the 120 mg regimen compared to the 60 mg regimen. Differences between the two age groups, however, were not statistically significant. In the same study, females showed a slight (15%) reduction in clearance when the dose was increased. Females showed higher AUCs and Cmax compared to males, but these differences were accounted for by differences in body weight between the two groups. When the data were analyzed using age as a variable, the results indicated that there were no significant differences in any of the pharmacokinetic variables of ISMN between older (≥65 years) and younger individuals (45-64 years). The results of this study, however, should be viewed with caution due to the small numbers of subjects in each age subgroup and consequently the lack of sufficient statistical power.
The following table summarizes key pharmacokinetic parameters of ISMN after single- and multiple-dose administration of ISMN as an oral solution or Isosorbide Mononitrate Tablets:
|
|
SINGLE-DOSE STUDIES |
MULTIPLE-DOSE STUDIES |
||
|
|
|
ISMN Extended- |
ISMN Extended- |
ISMN Extended- |
|
PARAMETER |
ISMN |
Release Tablet |
Release Tablet |
Release Tablet |
|
|
60 mg |
60 mg |
60mg |
120 mg |
|
Cmax (ng/mL) |
1242-1534 |
424-541 |
557-572 |
1151-1180 |
|
Tmax (hr) |
0.6-0.7 |
3.1-4.5 |
2.9-4.2 |
3.1-3.2 |
|
AUC (ng•hr/mL) |
8189- -8313 |
5990-7452 |
6625-7555 |
14241-16800 |
|
t1/2 (hr) |
4.8-5.1 |
6.3-6.6 |
6.2-6.3 |
6.2-6.4 |
|
Cl/F (mL/min) |
120-122 |
151-187 |
132-151 |
119-140 |
Food Effects
The influence of food on the bioavailability of ISMN after single-dose administration of Isosorbide Mononitrate Tablets 60 mg was evaluated in three different studies involving either a “light” breakfast or a high-calorie, high-fat breakfast. Results of these studies indicate that concomitant food intake may decrease the rate (increase in Tmax) but not the extent (AUC) of absorption of ISMN.
CLINICAL TRIALS
Controlled trials with Isosorbide Mononitrate Tablets have demonstrated antianginal activity following acute and chronic dosing. Administration of Isosorbide Mononitrate Tablets once daily, taken early in the morning on arising, provided at least 12 hours of antianginal activity.
In a placebo-controlled parallel study, 30, 60, 120, and 240 mg of Isosorbide Mononitrate Tablets were administered once daily for up to 6 weeks. Prior to randomization, all patients completed a 1- to 3-week single-blind placebo phase to demonstrate nitrate responsiveness and total exercise treadmill time reproducibility. Exercise tolerance tests using the Bruce Protocol were conducted prior to and at 4 and 12 hours after the morning dose on days 1, 7, 14, 28, and 42 of the double-blind period. Isosorbide Mononitrate Tablets 30 and 60 mg (only doses evaluated acutely) demonstrated a significant increase from baseline in total treadmill time relative to placebo at 4 and 12 hours after the administration of the first dose. At day 42, the 120 and 240 mg dose of Isosorbide Mononitrate Tablets demonstrated a significant increase in total treadmill time at 4 and 12 hours post-dosing, but by day 42, the 30 and 60 mg doses no longer were differentiable from placebo. Throughout chronic dosing, rebound was not observed in any Isosorbide Mononitrate treatment group.
Pooled data from two other trials, comparing Isosorbide Mononitrate Tablets 60 mg once daily, ISDN 30 mg QID, and placebo QID in patients with chronic stable angina using a randomized, double-blind, three-way crossover design found statistically significant increases in exercise tolerance times for Isosorbide Mononitrate Tablets compared to placebo at hours 4, 8, and 12 and to ISDN at hour 4. The increases in exercise tolerance on day 14, although statistically significant compared to placebo, were about half of that seen on day 1 of the trial.
INDICATIONS AND USAGE
Isosorbide Mononitrate Extended-Release Tablets, USP are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of oral isosorbide mononitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
CONTRAINDICATIONS
Isosorbide Mononitrate Tablets are contraindicated in patients who have shown hypersensitivity or idiosyncratic reactions to other nitrates or nitrites.
WARNINGS
Amplification of the vasodilatory effects of isosorbide mononitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
The benefits of ISMN in patients with acute myocardial infarction or congestive heart failure have not been established. Because the effects of isosorbide mononitrate are difficult to terminate rapidly, this drug is not recommended in these settings.
If isosorbide mononitrate is used in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia.
PRECAUTIONS
General
Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide mononitrate. This drug should therefore be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide mononitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence. The importance of these observations to the routine, clinical use of oral isosorbide mononitrate is not known.
Information for Patients
Patients should be told that the antianginal efficacy of Isosorbide Mononitrate Tablets can be maintained by carefully following the prescribed schedule of dosing. For most patients, this can be accomplished by taking the dose on arising.
As with other nitrates, daily headaches sometimes accompany treatment with isosorbide mononitrate. In patients who get these headaches, the headaches are a marker of the activity of the drug. Patients should resist the temptation to avoid headaches by altering the schedule of their treatment with isosorbide mononitrate, since loss of headache may be associated with simultaneous loss of antianginal efficacy. Aspirin or acetaminophen often successfully relieves isosorbide mononitrate-induced headaches with no deleterious effect on isosorbide mononitrate's antianginal efficacy.
Treatment with isosorbide mononitrate may be associated with light-headedness on standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
Drug Interactions
The vasodilating effects of isosorbide mononitrate may be additive with those of other vasodilators. Alcohol, in particular, has been found to exhibit additive effects of this variety.
Marked symptomatic orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used in combination. Dose adjustments of either class of agents may be necessary.
Drug/Laboratory Test Interactions
Nitrates and nitrites may interfere with the Zlatkis-Zak color reaction, causing falsely low readings in serum cholesterol determinations.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenicity was observed in rats exposed to isosorbide mononitrate in their diets at doses of up to 900 mg/kg/day for the first 6 months and 500 mg/kg/day for the remaining duration of a study in which males were dosed for up to 121 weeks and females were dosed for up to 137 weeks. No evidence of carcinogenicity was observed in mice exposed to isosorbide mononitrate in their diets for up to 104 weeks at doses of up to 900 mg/kg/day.
Isosorbide mononitrate did not produce gene mutations (Ames test, mouse lymphoma test) or chromosome aberrations (human lymphocyte and mouse micronucleus tests) at biologically relevant concentrations.
No effects on fertility were observed in a study in which male and female rats were administered doses of up to 750 mg/kg/day beginning, in males, 9 weeks prior to mating, and in females, 2 weeks prior to mating.
Pregnancy Teratogenic Effects: Pregnancy Category B
In studies designed to detect effects of isosorbide mononitrate on embryo-fetal development, doses of up to 240 or 248 mg/kg/day, administered to pregnant rats and rabbits, were unassociated with evidence of such effects. These animal doses are about 100 times the maximum recommended human dose (120 mg in a 50 kg woman) when comparison is based on body weight; when comparison is based on body surface area, the rat dose is about 17 times the human dose and the rabbit dose is about 38 times the human dose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Isosorbide Mononitrate Tablets should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Neonatal survival and development and incidence of stillbirths were adversely affected when pregnant rats were administered oral doses of 750 (but not 300) mg isosorbide mononitrate/kg/day during late gestation and lactation. This dose (about 312 times the human dose when comparison is based on body weight and 54 times the human dose when comparison is based on body surface area) was associated with decreases in maternal weight gain and motor activity and evidence of impaired lactation.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ISMN is administered to a nursing mother.
Geriatric Use
Clinical studies of Isosorbide Mononitrate Tablets did not include sufficient information on patients age 65 and over to determine if they respond differently from younger patients. Other reported clinical experience for Isosorbide Mononitrate Tablets has not identified differences in response between elderly and younger patients. Clinical experience for organic nitrates reported in the literature identified a potential for severe hypotension and increased sensitivity to nitrates in the elderly. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Elderly patients may have reduced baroreceptor function and may develop severe orthostatic hypotension when vasodilators are used. Isosorbide Mononitrate Tablets should therefore be used with caution in elderly patients who may be volume depleted, on multiple medications, or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide mononitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
Elderly patients may be more susceptible to hypotension and may be at a greater risk of falling at therapeutic doses of nitroglycerin.
Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy, particularly in the elderly.
ADVERSE REACTIONS
The table below shows the frequencies of the adverse events that occurred in >5% of the subjects in three placebo-controlled North American studies in which patients in the active treatment arm received 30 mg, 60 mg, 120 mg, or 240 mg of isosorbide mononitrate as Isosorbide Mononitrate Tablets once daily. In parentheses, the same table shows the frequencies with which these adverse events were associated with the discontinuation of treatment. Overall, 8% of the patients who received 30 mg, 60 mg, 120 mg, or 240 mg of isosorbide mononitrate in the three placebo-controlled North American studies discontinued treatment because of adverse events. Most of these discontinued because of headache. Dizziness was rarely associated with withdrawal from these studies. Since headache appears to be a dose-related adverse effect and tends to disappear with continued treatment, it is recommended that isosorbide mononitrate tablets treatment be initiated at low doses for several days before being increased to desired levels.
|
Three Controlled North American Studies |
|||||
|
Dose |
Placebo |
30 mg |
60 mg |
120 mg** |
240 mg** |
|
Patients |
96 |
60 |
102 |
65 |
65 |
|
Headache |
15% (0%) |
38% (5%) |
51% (8%) |
42% (5%) |
57% (8%) |
|
Dizziness |
4% (0%) |
8% (0%) |
11% (1%) |
9% (2%) |
9% (2%) |
In addition, the three North American trials were pooled with 11 controlled trials conducted in Europe. Among the 14 controlled trials, a total of 711 patients were randomized to Isosorbide Mononitrate Tablets. When the pooled data were reviewed, headache and dizziness were the only adverse events that were reported by >5% of patients. Other adverse events, each reported by ≤5% of exposed patients, and in many cases of uncertain relation to drug treatment, were:
Autonomic Nervous System Disorders: Dry mouth, hot flushes.
Body as a Whole: Asthenia, back pain, chest pain, edema, fatigue, fever, flu-like symptoms, malaise, rigors.
Cardiovascular Disorders, General: Cardiac failure, hypertension, hypotension.
Central and Peripheral Nervous System Disorders: Dizziness, headache, hypoesthesia, migraine, neuritis, paresis, paresthesia, ptosis, tremor, vertigo.
Gastrointestinal System Disorders: Abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gastric ulcer, gastritis, glossitis, hemorrhagic gastric ulcer, hemorrhoids, loose stools, melena, nausea, vomiting.
Hearing and Vestibular Disorders: Earache, tinnitus, tympanic membrane perforation.
Heart Rate and Rhythm Disorders: Arrhythmia, arrhythmia atrial, atrial fibrillation, bradycardia, bundle branch block, extrasystole, palpitation, tachycardia, ventricular tachycardia.
Liver and Biliary System Disorders: SGOT increase, SGPT increase.
Metabolic and Nutritional Disorders: Hyperuricemia, hypokalemia.
Musculoskeletal System Disorders: Arthralgia, frozen shoulder, muscle weakness, musculoskeletal pain, myalgia, myositis, tendon disorder, torticollis.
Myo-, Endo-, Pericardial, and Valve Disorders: Angina pectoris aggravated, heart murmur, heart sound abnormal, myocardial infarction, Q wave abnormality.
Platelet, Bleeding, and Clotting Disorders: Purpura, thrombocytopenia.
Psychiatric Disorders: Anxiety, concentration impaired, confusion, decreased libido, depression, impotence, insomnia, nervousness, paroniria, somnolence.
Red Blood Cell Disorder: Hypochromic anemia.
Reproductive Disorders, Female: Atrophic vaginitis, breast pain.
Resistance Mechanism Disorders: Bacterial infection, moniliasis, viral infection.
Respiratory System Disorders: Bronchitis, bronchospasm, coughing, dyspnea, increased sputum, nasal congestion, pharyngitis, pneumonia, pulmonary infiltration, rales, rhinitis, sinusitis.
Skin and Appendages Disorders: Acne, hair texture abnormal, increased sweating, pruritus, rash, skin nodule.
Urinary System Disorders: Polyuria, renal calculus, urinary tract infection.
Vascular (Extracardiac) Disorders: Flushing, intermittent claudication, leg ulcer, varicose vein.
Vision Disorders: Conjunctivitis, photophobia, vision abnormal.
In addition, the following spontaneous adverse event has been reported during the marketing of isosorbide mononitrate: syncope.
To report SUSPECTED ADVERSE REACTIONS, contact West-ward Pharmaceutical Corp. at 1-877-233-2001, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Hemodynamic Effects
The ill effects of isosorbide mononitrate overdose are generally the results of isosorbide mononitrate's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures and death.
Laboratory determinations of serum levels of isosorbide mononitrate and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of isosorbide mononitrate overdose.
There are no data suggesting what dose of isosorbide mononitrate is likely to be life threatening in humans. In rats and mice, there is significant lethality at doses of 2000 mg/kg and 3000 mg/kg, respectively.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of isosorbide mononitrate. In particular, dialysis is known to be ineffective in removing isosorbide mononitrate from the body.
No specific antagonist to the vasodilator effects of isosorbide mononitrate is known, and no intervention has been subject to controlled study as a therapy of isosorbide mononitrate overdose. Because the hypotension associated with isosorbide mononitrate overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward an increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of isosorbide mononitrate overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia
Methemoglobinemia has been reported in patients receiving other organic nitrates, and it probably could also occur as a side effect of isosorbide mononitrate. Certainly, nitrate ions liberated during metabolism of isosorbide mononitrate can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity, however, and even assuming that the nitrate moiety of isosorbide mononitrate is quantitatively applied to oxidation of hemoglobin, about 2 mg/kg of isosorbide mononitrate should be required before any of these patients manifest clinically significant (≥10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of isosorbide mononitrate. In one study in which 36 patients received 2 to 4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/hr (equivalent, in total administered dose of nitrate ions, to 7.8-11.1 mg of isosorbide mononitrate per hour), the average methemoglobin level measured was 0.2%; this was comparable to that observed in parallel patients who received placebo.
Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the affected patients had been thought to be unusually susceptible.
Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial p02. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1 to 2 mg/kg intravenously.
DOSAGE AND ADMINISTRATION
The recommended starting dose of Isosorbide Mononitrate Tablets is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once daily. After several days, the dosage may be increased to 120 mg (given as a single 120 mg tablet or as two 60 mg tablets) once daily. Rarely, 240 mg may be required. The daily dose of Isosorbide Mononitrate Tablets should be taken in the morning on arising. Isosorbide Mononitrate Extended-Release Tablets should not be chewed or crushed and should be swallowed together with a half-glassful of fluid. Do not break the 30 mg tablet.
HOW SUPPLIED
Isosorbide Mononitrate Extended-Release Tablets, 30 mg: Film-Coated Light Rose, Oval Tablets; Debossed "WW" on one Scored side and "30" on the other Scored side.
Unit dose packages of 100 (10 x 10) NDC 68084-435-01
Isosorbide Mononitrate Extended-Release Tablets, 60 mg: Film-Coated Light Yellow, Oval Tablets; Debossed "WW" on one Scored side and "60" on the other Scored side.
Unit dose packages of 100 (10 x 10) NDC 68084-436-01
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section) contain drug product from West-ward Pharmaceutical Corp. as follows:
(30 mg / 100 UD) NDC 68084-435-01 packaged from NDC 0143-2230
(60 mg / 100 UD) NDC 68084-436-01 packaged from NDC 0143-2260
Packaged and Distributed by:
American Health Packaging
Columbus, OH 43217
8243601/1112
| ISOSORBIDE MONONITRATE
isosorbide mononitrate tablet, film coated, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ISOSORBIDE MONONITRATE
isosorbide mononitrate tablet, film coated, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Health Packaging (929561009) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Health Packaging | 929561009 | REPACK(68084-435, 68084-436) | |