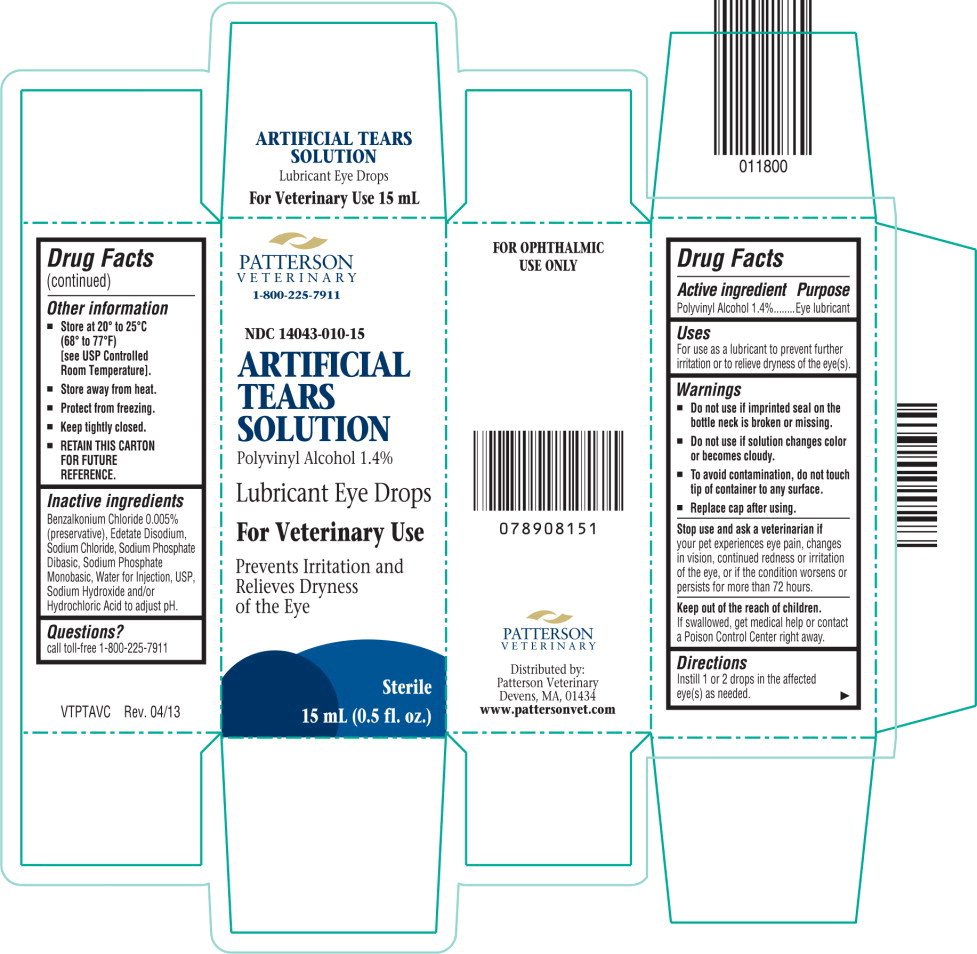
ARTIFICIAL TEARS- polyvinyl alcohol, unspecified solution/ drops
Patterson Veterinary
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Warnings
- Do not use if imprinted seal on the bottle neck is broken or missing.
- Do not use it solution changes color or becomes cloudy.
- To avoid contamination, do not touch tip of container to any surface.
- Replace cap after using.
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Store away from heat.
- Protect from freezing.
- Keep tightly closed.
- RETAIN THIS CARTON FOR FUTURE REFERENCE.
Inactive ingredients
Benzalkonium Chloride 0.005% (preservative), Edetate Disodium, Sodium Chloride, Sodium Phosphate Dibasic, Sodium Phosphate Monobasic, Water for Injection, USP, Sodium Hydroxide and/or Hydrochloric Acid to adjust pH.
| ARTIFICIAL TEARS
polyvinyl alcohol, unspecified solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Patterson Veterinary (006962500) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | MANUFACTURE, ANALYSIS, STERILIZE, PACK, LABEL | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avantor Performance Materials International, Inc. | 152791026 | API MANUFACTURE | |
Revised: 2/2022
Document Id: e81e27c5-b7d4-4a0d-9303-eea016dfb40c
Set id: eda3f20d-940c-44ed-9817-67a439dd5330
Version: 4
Effective Time: 20220210
Patterson Veterinary