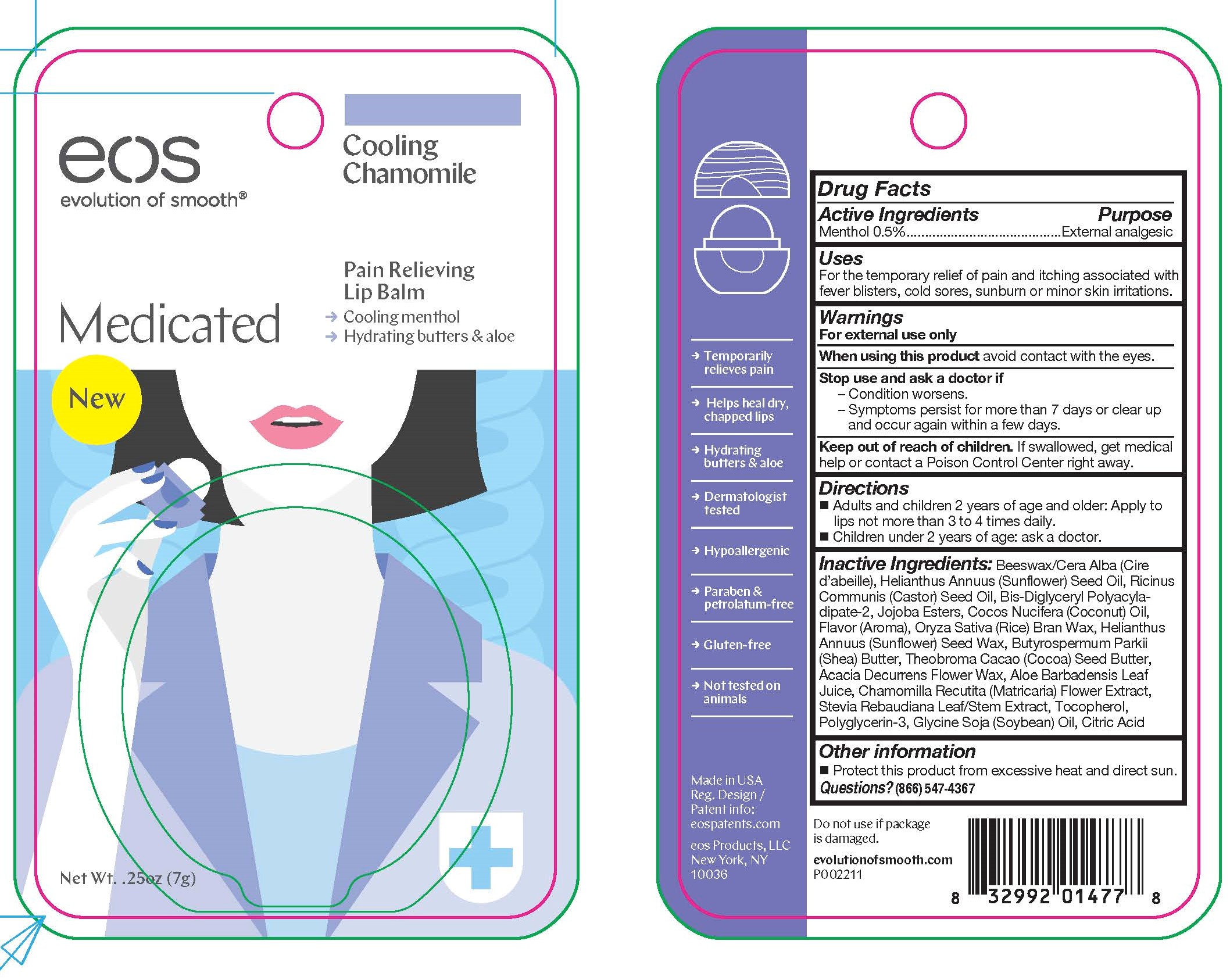
EOS MEDICATED COOLING CHAMOMILE- menthol stick
eos Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only
Active ingredients Purpose
Menthol 0.5% External analgesic
Uses
For the temporary relief of pain and itching associated with fever blisters, cold sores, sunburn or minor skin irritations.
When using this product avoid contact with the eyes.
Stop use and ask a doctor if
-Condition worsens.
-Symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: Apply to lips not more than 3 or 4 times daily.
- Children under 2 years of age: ask a doctor.
Inactive Ingredients:
Beeswax/Cera Alba (Cire d’abeille), Helianthus Annuus (Sunflower) Seed Oil, Ricinus Communis (Castor) Seed Oil, Bis-Diglyceryl Polyacyladipate-
2, Jojoba Esters, Cocos Nucifera (Coconut) Oil, Flavor (Aroma), Oryza Sativa (Rice) Bran Wax, Helianthus Annuus (Sunflower) Seed Wax, Butyrospermum Parkii (Shea) Butter, Theobroma Cacao (Cocoa) Seed Butter, Acacia Decurrens Flower Wax, Aloe Barbadensis Leaf
Juice, Chamomilla Recutita (Matricaria) Flower Extract, Stevia Rebaudiana Leaf/Stem Extract, Tocopherol, Polyglycerin-3, Glycine Soja (Soybean) Oil, Citric Acid
Other information
- Protect this product from excessive heat and direct sun.
Questions?
(866) 547-4367
Principal Display Pantel - .025 oz (7 g)
eos
Medicated Cooling Chamomile
Lip Balm
Net wt. 0.25 oz (7 g)