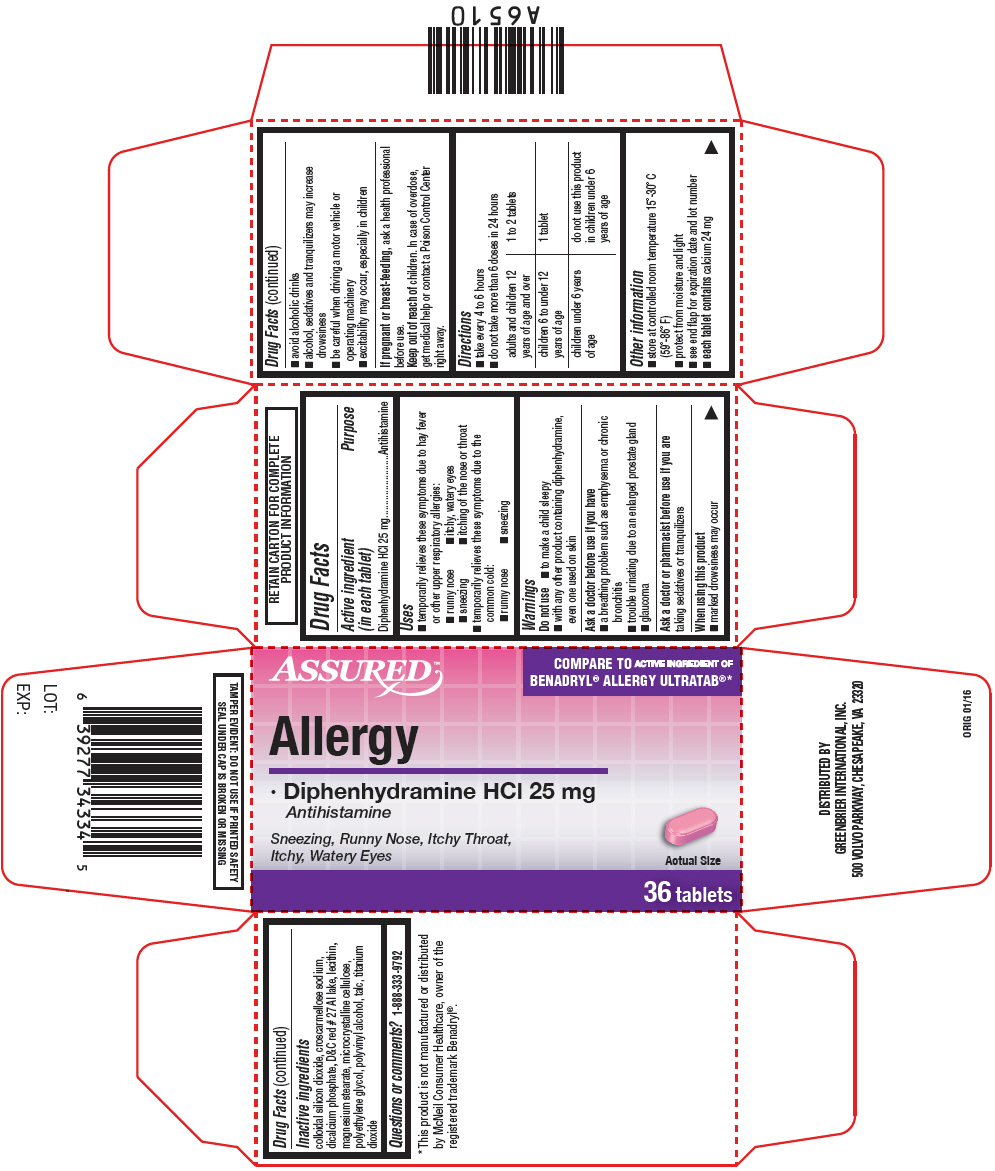
ASSURED ALLERGY RELIEF- diphenhydramine hydrochloride tablet, film coated
Spirit Pharmaceutical LLC
----------
Assured™ Allergy Relief
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years of age and over | 1 to 2 tablets |
| children 6 to under 12 years of age | 1 tablet |
| children under 6 years of age | do not use this product in children under 6 years of age |
Other information
- store at controlled room temperature 15°-30° C (59°-86° F)
- protect from moisture and light
- see end flap for expiration date and lot number
- each tablet contains calcium 24 mg
| ASSURED ALLERGY RELIEF
diphenhydramine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Spirit Pharmaceutical LLC (179621011) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Elysium Pharmaceutical Ltd | 915664486 | manufacture(68210-1002) | |
Revised: 12/2023
Document Id: 0bf31911-9c21-24fc-e063-6394a90a6386
Set id: ec0863eb-2a59-4968-b96e-a3b02b1f241b
Version: 7
Effective Time: 20231207
Spirit Pharmaceutical LLC