PFIZERPEN- penicillin g potassium powder, for solution
Roerig
----------
Buffered
PFIZERPEN
(penicillin G potassium)
for Injection
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Pfizerpen® and other antibacterial drugs, Pfizerpen should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Buffered Pfizerpen (penicillin G potassium) for Injection is a sterile, pyrogen-free powder for reconstitution. Buffered Pfizerpen for Injection is an antibacterial agent for intramuscular, continuous intravenous drip, intrapleural or other local infusion, and intrathecal administration.
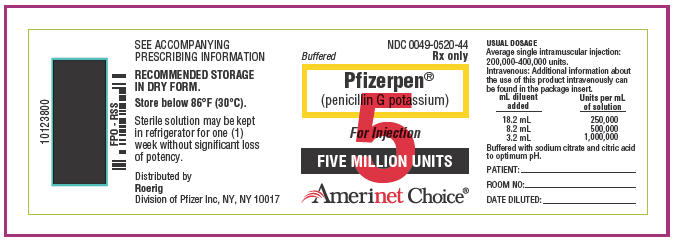
Each million units contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq).
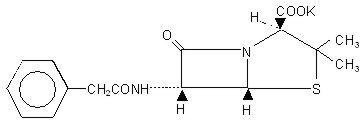
Chemically, Pfizerpen is monopotassium 3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate. It has a molecular weight of 372.48 and the following chemical structure:
Formula
C16H17KN2O4S
Penicillin G potassium is a colorless or white crystal, or a white crystalline powder which is odorless, or practically so, and moderately hygroscopic. Penicillin G potassium is very soluble in water. The pH of the reconstituted product is between 6.0–8.5.
CLINICAL PHARMACOLOGY
Aqueous penicillin G is rapidly absorbed following both intramuscular and subcutaneous injection. Initial blood levels following parenteral administration are high but transient. Penicillins bind to serum proteins, mainly albumin. Therapeutic levels of the penicillins are easily achieved under normal circumstances in extracellular fluid and most other body tissues. Penicillins are distributed in varying degrees into pleural, pericardial, peritoneal, ascitic, synovial, and interstitial fluids. Penicillins are excreted in breast milk. Penetration into the cerebrospinal fluid, eyes, and prostate is poor. Penicillins are rapidly excreted in the urine by glomerular filtration and active tubular secretion, primarily as unchanged drug. Approximately 60 percent of the total dose of 300,000 units is excreted in the urine within this 5-hour period. For this reason, high and frequent doses are required to maintain the elevated serum levels desirable in treating certain severe infections in individuals with normal kidney function. In neonates and young infants, and in individuals with impaired kidney function, excretion is considerably delayed.
Microbiology
Penicillin G exerts a bactericidal action against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell wall mucopeptide rendering the cell wall osmotically unstable. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. While in vitro studies have demonstrated the susceptibility of most strains of the following organisms, clinical efficacy for infections other than those included in the INDICATIONS AND USAGE section has not been documented. Penicillin G exerts high in vitro activity against staphylococci (except penicillinase-producing strains), streptococci (groups A, C, G, H, L, and M), and pneumococci. Other organisms susceptible to penicillin G are N. gonorrhoeae, Corynebacterium diphtheriae, Bacillus anthracis, Clostridia, Actinomyces bovis, Streptobacillus moniliformis, Listeria monocytogenes and Leptospira. Treponema pallidum is extremely sensitive to the bactericidal action of penicillin G. Some species of gram-negative bacilli are sensitive to moderate to high concentrations of the drug obtained with intravenous administration. These include most strains of Escherichia coli; all strains of Proteus mirabilis, Salmonella and Shigella; and some strains of Aerobacter aerogenes and Alcaligenes faecalis.
Penicillin acts synergistically with gentamicin or tobramycin against many strains of enterococci.
Susceptibility Testing
Penicillin G Susceptibility Powder or 10 units Penicillin G Susceptibility Discs may be used to determine microbial susceptibility to penicillin G using one of the following standard methods recommended by the National Committee for Laboratory Standards:
M2-A3, "Performance Standards for Antimicrobial Disk Susceptibility Tests"
M7-A, "Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically"
M11-A, "Reference Agar Dilution Procedure for Antimicrobial Susceptibility Testing of Anaerobic Bacteria"
M17-P, "Alternative Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria"
Tests should be interpreted by the following criteria:
| Zone Diameter, nearest whole mm | |||
|---|---|---|---|
| Susceptible | Moderately Susceptible | Resistant | |
| Staphylococci | ≥29 | – | ≤28 |
| N. gonorrhoeae | ≥20 | – | ≤19 |
| Enterococci | – | ≥15 | ≤14 |
| Non-enterococcal streptococci and L. monocytogenes | ≥28 | 20–27 | ≤19 |
| Approximate MIC Correlates | ||
|---|---|---|
| Susceptible | Resistant | |
| Staphylococci | ≤0.1 µg/mL | β-lactamase |
| N. gonorrhoeae | ≤0.1 µg/mL | β-lactamase |
| Enterococci | – | ≥16 µg/mL |
| Non-enterococcal streptococci and L. monocytogenes | ≤0.12 µg/mL | ≥ 4 µg/mL |
Interpretations of susceptible, intermediate, and resistant correlate zone size diameters with MIC values. A laboratory report of "susceptible" indicates that the suspected causative microorganism most likely will respond to therapy with penicillin G. A laboratory report of "resistant" indicates that the infecting microorganism most likely will not respond to therapy. A laboratory report of "moderately susceptible" indicates that the microorganism is most likely susceptible if a high dosage of penicillin G is used, or if the infection is such that high levels of penicillin G may be attained, as in urine. A report of "intermediate" using the disk diffusion method may be considered an equivocal result, and dilution tests may be indicated.
Control organisms are recommended for susceptibility testing. Each time the test is performed the following organisms should be included. The range for zones of inhibition is shown below:
| Control Organism | Zone of Inhibition Range |
|---|---|
| Staphylococcus aureus (ATCC 25923) | 27–35 |
INDICATIONS AND USAGE
Aqueous penicillin G (parenteral) is indicated in the therapy of severe infections caused by penicillin G-susceptible microorganisms when rapid and high penicillin levels are required in the conditions listed below. Therapy should be guided by bacteriological studies (including susceptibility tests) and by clinical response.
The following infections will usually respond to adequate dosage of aqueous penicillin G (parenteral):
- Streptococcal infections.
NOTE: Streptococci in groups A, C, H, G, L, and M are very sensitive to penicillin G. Some group D organisms are sensitive to the high serum levels obtained with aqueous penicillin G.
Aqueous penicillin G (parenteral) is the penicillin dosage form of choice for bacteremia, empyema, severe pneumonia, pericarditis, endocarditis, meningitis, and other severe infections caused by sensitive strains of the gram-positive species listed above.
- Pneumococcal infections.
- Staphylococcal infections–penicillin G sensitive.
-
Other infections:
- Anthrax.
- Actinomycosis.
- Clostridial infections (including tetanus).
- Diphtheria (to prevent carrier state).
- Erysipeloid (Erysipelothrix insidiosa) endocarditis.
- Fusospirochetal infections–severe infections of the oropharynx (Vincent's), lower respiratory tract and genital area due to Fusobacterium fusiformisans spirochetes.
- Gram-negative bacillary infections (bacteremias)–(E. coli, A. aerogenes, A. faecalis, Salmonella, Shigella and P. mirabilis).
- Listeria infections (Listeria monocytogenes).
- Meningitis and endocarditis.
- Pasteurella infections (Pasteurella multocida).
- Bacteremia and meningitis.
- Rat-bite fever (Spirillum minus or Streptobacillus moniliformis).
- Gonorrheal endocarditis and arthritis (N. gonorrhoeae).
- Syphilis (T. pallidum) including congenital syphilis.
- Meningococcic meningitis.
Although no controlled clinical efficacy studies have been conducted, aqueous crystalline penicillin G for injection and penicillin G procaine suspension have been suggested by the American Heart Association and the American Dental Association for use as part of a combined parenteral-oral regimen for prophylaxis against bacterial endocarditis in patients with congenital heart disease or rheumatic, or other acquired valvular heart disease when they undergo dental procedures and surgical procedures of the upper respiratory tract.1 Since it may happen that alpha hemolytic streptococci relatively resistant to penicillin may be found when patients are receiving continuous oral penicillin for secondary prevention of rheumatic fever, prophylactic agents other than penicillin may be chosen for these patients and prescribed in addition to their continuous rheumatic fever prophylactic regimen.
NOTE: When selecting antibiotics for the prevention of bacterial endocarditis, the physician or dentist should read the full joint statement of the American Heart Association and the American Dental Association.1
To reduce the development of drug-resistant bacteria and maintain effectiveness of Pfizerpen and other antibacterial drugs, Pfizerpen should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
A history of a previous hypersensitivity reaction to any penicillin is a contraindication.
WARNINGS
Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before initiating therapy with any penicillin, careful inquiry should be made concerning previous hypersensitivity reactions to penicillin, cephalosporins, or other allergens. If an allergic reaction occurs, the drug should be discontinued and the appropriate therapy instituted. Serious anaphylactoid reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management including intubation, should also be administered as indicated.
PRECAUTIONS
General
Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma.
Prescribing Pfizerpen in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including Pfizerpen should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Pfizerpen is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Pfizerpen or other antibacterial drugs in the future.
Intramuscular Therapy
Care should be taken to avoid intravenous or accidental intraarterial administration, or injection into or near major peripheral nerves or blood vessels, since such injections may produce neurovascular damage. Particular care should be taken with IV administration because of the possibility of thrombophlebitis.
In streptococcal infections, therapy must be sufficient to eliminate the organism (10 days minimum), otherwise the sequelae of streptococcal disease may occur. Cultures should be taken following the completion of treatment to determine whether streptococci have been eradicated.
The use of antibiotics may result in overgrowth of nonsusceptible organisms. Constant observation of the patient is essential. If new infections due to bacteria or fungi appear during therapy, the drug should be discontinued and appropriate measures taken. Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life threatening and amenable only to penicillin therapy.
Aqueous penicillin G by the intravenous route in high doses (above 10 million units) should be administered slowly because of the adverse effects of electrolyte imbalance from either the potassium or sodium content of the penicillin. Penicillin G potassium contains 1.7 mEq potassium and 0.3 mEq sodium per million units. The patient's renal, cardiac, and vascular status should be evaluated and if impairment of function is suspected or known to exist a reduction in the total dosage should be considered. Frequent evaluation of electrolyte balance, renal and hematopoietic function is recommended during therapy when high doses of intravenous aqueous penicillin G are used.
Laboratory Tests
In prolonged therapy with penicillin, periodic evaluation of the renal, hepatic, and hematopoietic systems is recommended for organ system dysfunction. This is particularly important in prematures, neonates and other infants, and when high doses are used.
Positive Coomb's tests have been reported after large intravenous doses.
Monitor serum potassium and implement corrective measures when necessary.
When treating gonococcal infections in which primary and secondary syphilis are suspected, proper diagnostic procedures, including dark field examinations, should be done before receiving penicillin and monthly serological tests made for at least four months. All cases of penicillin treated syphilis should receive clinical and serological examinations every six months for two to three years.
In suspected staphylococcal infections, proper laboratory studies, including susceptibility tests, should be performed.
In streptococcal infections, cultures should be taken following completion of treatment to determine whether streptococci have been eradicated. Therapy must be sufficient to eliminate the organism (a minimum of 10 days), otherwise the sequelae of streptococcal disease (e.g., endocarditis, rheumatic fever) may occur.
Drug Interactions
Concurrent administration of bacteriostatic antibiotics (e.g., erythromycin, tetracycline) may diminish the bactericidal effects of penicillins by slowing the rate of bacterial growth. Bactericidal agents work most effectively against the immature cell wall of rapidly proliferating microorganisms. This has been demonstrated in vitro; however, the clinical significance of this interaction is not well documented. There are few clinical situations in which the concurrent use of "static" and "cidal" antibiotics are indicated. However, in selected circumstances in which such therapy is appropriate, using adequate doses of antibacterial agents and beginning penicillin therapy first, should minimize the potential for interaction.
Penicillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins.
Displacement of penicillin from plasma protein binding sites will elevate the level of free penicillin in the serum.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No information on long-term studies are available on the carcinogenesis, mutagenesis, or the impairment of fertility with the use of penicillins.
Pregnancy Category B
Teratogenic Effects
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
ADVERSE REACTIONS
Penicillin is a substance of low toxicity but does have a significant index of sensitization. The following hypersensitivity reactions have been reported: skin rashes ranging from maculopapular eruptions to exfoliative dermatitis; urticaria; and reactions resembling serum sickness, including chills, fever, edema, arthralgia and prostration. Severe and occasionally fatal anaphylaxis has occurred (see WARNINGS).
Hemolytic anemia, leucopenia, thrombocytopenia, nephropathy, and neuropathy are rarely observed adverse reactions and are usually associated with high intravenous dosage. Patients given continuous intravenous therapy with penicillin G potassium in high dosage (10 million to 100 million units daily) may suffer severe or even fatal potassium poisoning, particularly if renal insufficiency is present. Hyperreflexia, convulsions, and coma may be indicative of this syndrome.
Cardiac arrhythmias and cardiac arrest may also occur. (High dosage of penicillin G sodium may result in congestive heart failure due to high sodium intake.)
The Jarisch-Herxheimer reaction has been reported in patients treated for syphilis.
OVERDOSAGE
Neurological adverse reactions, including convulsions, may occur with the attainment of high CSF levels of beta-lactams. In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required.
Penicillin G potassium is hemodialyzable.
DOSAGE AND ADMINISTRATION
Severe infections due to Susceptible Strains of Streptococci, Pneumococci, and Staphylococci–bacteremia, pneumonia, endocarditis, pericarditis, empyema, meningitis, and other severe infections–a minimum of 5 million units daily.
Syphilis–Aqueous penicillin G may be used in the treatment of acquired and congenital syphilis, but because of the necessity of frequent dosage, hospitalization is recommended. Dosage and duration of therapy will be determined by age of patient and stage of the disease.
Gonorrheal endocarditis–a minimum of 5 million units daily.
Meningococcic meningitis–1–2 million units intramuscularly every 2 hours, or continuous IV drip of 20–30 million units/day.
Actinomycosis–1–6 million units/day for cervicofacial cases; 10–20 million units/day for thoracic and abdominal disease.
Clostridial infections–20 million units/day; penicillin is adjunctive therapy to antitoxin.
Fusospirochetal infections–severe infections of oropharynx, lower respiratory tract, and genital area–5–10 million units/day.
Rat-bite fever (Spirillum minus or Streptobacillus moniliformis)–12–15 million units/day for 3–4 weeks.
Listeria infections (Listeria monocytogenes)
Neonates–500,000 to 1 million units/day.
Adults with meningitis–15–20 million units/day for 2 weeks.
Adults with endocarditis–15–20 million units/day for 4 weeks.
Pasteurella infections (Pasteurella multocida)
Bacteremia and meningitis 4–6 million units/day for 2 weeks.
Erysipeloid (Erysipelothrix insidiosa)
Endocarditis–2–20 million units/day for 4–6 weeks.
Gram-negative bacillary infections (E. coli, Enterobacter aerogenes, A. faecalis, Salmonella, Shigella and Proteus mirabilis)
Bacteremia–20–80 million units/day.
Diphtheria (carrier state)–300,000–400,000 units of penicillin/day in divided doses for 10–12 days.
Anthrax–A minimum of 5 million units of penicillin/day in divided doses until cure is effected.
For prophylaxis against bacterial endocarditis1 in patients with congenital heart disease or rheumatic or other acquired valvular heart disease, when undergoing dental procedures or surgical procedures of the upper respiratory tract, use a combined parenteral-oral regimen. One million units of aqueous crystalline penicillin G (30,000 units/kg in children) intramuscularly, mixed with 600,000 units procaine penicillin G (600,000 units for children) should be given one-half to one hour before the procedure. Oral penicillin V (phenoxymethyl penicillin), 500 mg for adults or 250 mg for children less than 60 lb, should be given every 6 hours for 8 doses. Doses for children should not exceed recommendations for adults for a single dose or for a 24 hour period.
Reconstitution
The following table shows the amount of solvent required for solution of various concentrations:
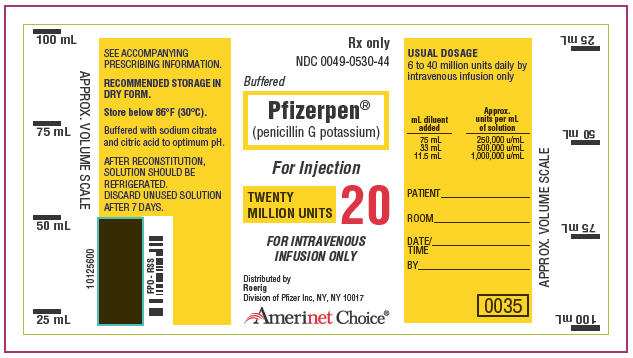
| Approx. Desired Concentration (units/mL) | Approx. Volume (mL) 1,000,000 units | Solvent for Vial of 5,000,000 units | Infusion Only 20,000,000 units |
|---|---|---|---|
| 50,000 | 20.0 | – | – |
| 100,000 | 10.0 | – | – |
| 250,000 | 4.0 | 18.2 | 75.0 |
| 500,000 | 1.8 | 8.2 | 33.0 |
| 750,000 | – | 4.8 | – |
| 1,000,000 | – | 3.2 | 11.5 |
When the required volume of solvent is greater than the capacity of the vial, the penicillin can be dissolved by first injecting only a portion of the solvent into the vial, then withdrawing the resultant solution and combining it with the remainder of the solvent in a larger sterile container.
Buffered Pfizerpen (penicillin G potassium) for Injection is highly water soluble. It may be dissolved in small amounts of Water for Injection, or Sterile Isotonic Sodium Chloride Solution for Parenteral Use. All solutions should be stored in a refrigerator. When refrigerated, penicillin solutions may be stored for seven days without significant loss of potency.
Buffered Pfizerpen for Injection may be given intramuscularly or by continuous intravenous drip for dosages of 500,000, 1,000,000, or 5,000,000 units. It is also suitable for intrapleural, intraarticular, and other local instillations.
THE 20,000,000 UNIT DOSAGE MAY BE ADMINISTERED BY INTRAVENOUS INFUSION ONLY.
(1) Intramuscular Injection
Keep total volume of injection small. The intramuscular route is the preferred route of administration. Solutions containing up to 100,000 units of penicillin per mL of diluent may be used with a minimum of discomfort. Greater concentration of penicillin G per mL is physically possible and may be employed where therapy demands. When large dosages are required, it may be advisable to administer aqueous solutions of penicillin by means of continuous intravenous drip.
(2) Continuous Intravenous Drip
Determine the volume of fluid and rate of its administration required by the patient in a 24 hour period in the usual manner for fluid therapy, and add the appropriate daily dosage of penicillin to this fluid. For example, if an adult patient requires 2 liters of fluid in 24 hours and a daily dosage of 10 million units of penicillin, add 5 million units to 1 liter and adjust the rate of flow so the liter will be infused in 12 hours.
(3) Intrapleural or Other Local Infusion
If fluid is aspirated, give infusion in a volume equal to ¼ or ½ the amount of fluid aspirated, otherwise, prepare as for intramuscular injection.
(4) Intrathecal Use
The intrathecal use of penicillin in meningitis must be highly individualized. It should be employed only with full consideration of the possible irritating effects of penicillin when used by this route. The preferred route of therapy in bacterial meningitides is intravenous, supplemented by intramuscular injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Sterile solution may be left in refrigerator for one week without significant loss of potency.
HOW SUPPLIED
Buffered Pfizerpen (penicillin G potassium) for Injection is available in vials containing respectively 5,000,000 units × 10's (NDC 0049-0520-44) and 20,000,000 units × 1's (NDC 0049-0530-44); buffered with sodium citrate and citric acid to an optimum pH.
Each million units contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq).
Reference
- American Heart Association, 1977. Prevention of bacterial endocarditis.
Circulation. 56:139A–143A.
| PFIZERPEN
penicillin g potassium powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PFIZERPEN
penicillin g potassium powder, for solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Roerig (829076996) |