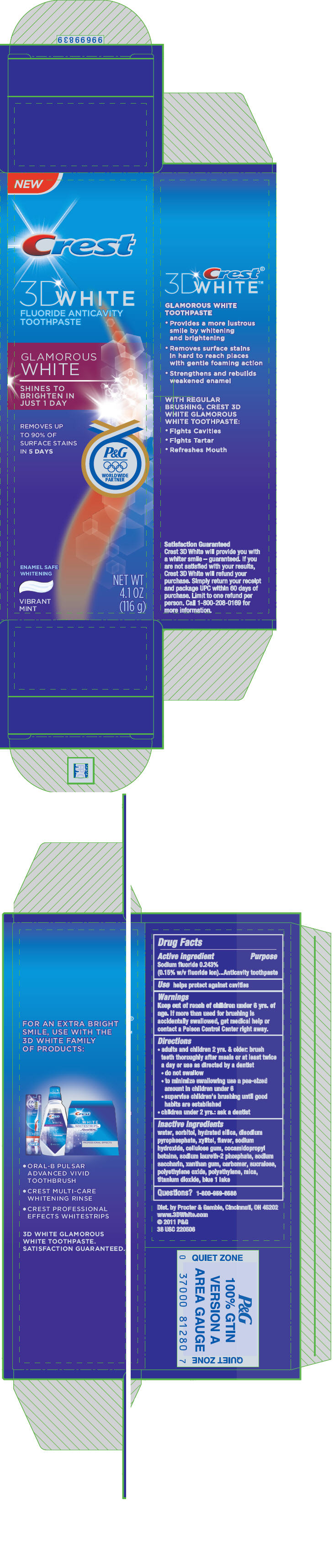
CREST 3D WHITE GLAMOROUS WHITE- sodium fluoride paste, dentifrice
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crest
® 3D White™
Glamorous White
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
Inactive ingredients
water, sorbitol, hydrated silica, disodium pyrophosphate, xylitol, flavor, sodium hydroxide, cellulose gum, cocamidopropyl betaine, sodium laureth-2 phosphate, sodium saccharin, xanthan gum, carbomer, sucralose, polyethylene oxide, polyethylene, mica, titanium dioxide, blue 1 lake
| CREST 3D WHITE
GLAMOROUS WHITE
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
Revised: 10/2016
Document Id: 3f113700-02a3-2219-e054-00144ff88e88
Set id: eaed97cd-33c0-4d21-93b3-989b2776e49d
Version: 2
Effective Time: 20161017
Procter & Gamble Manufacturing Company