CLARITHROMYCIN- clarithromycin tablet
REMEDYREPACK INC.
----------
DESCRIPTION
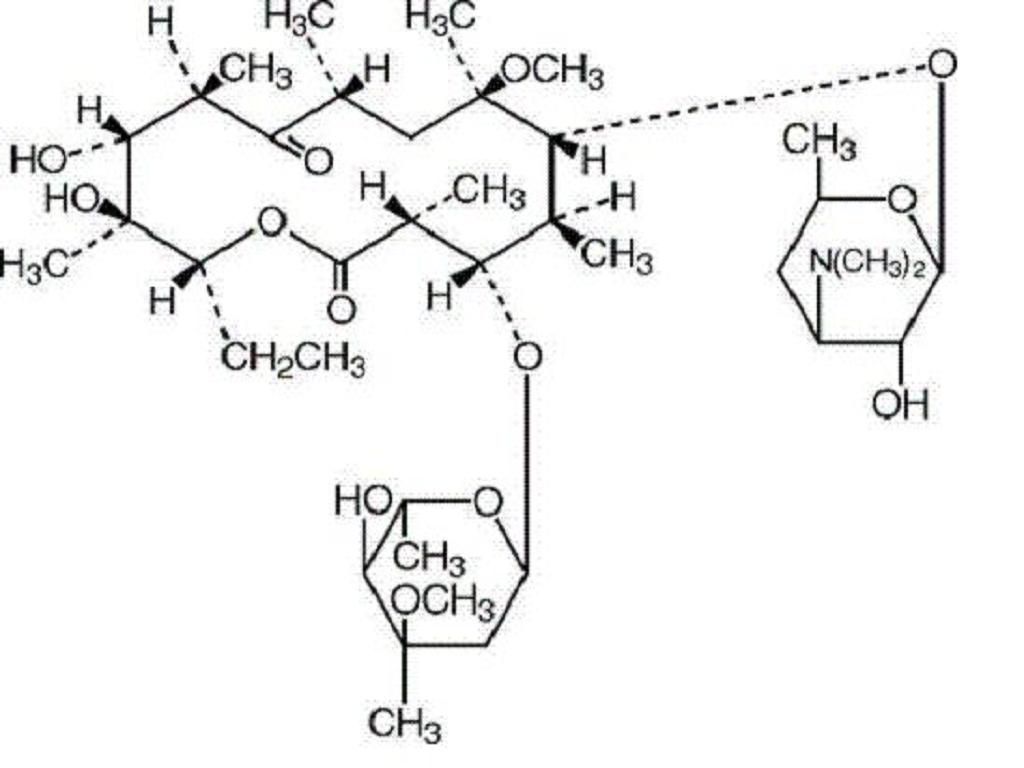
Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.95. The structural formula is:
Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water.
Clarithromycin is available as immediate-release tablets, USP and granules for oral suspension USP.
Each light yellow capsule-shaped film-coated immediate-release clarithromycin tablet contains 250 mg or 500 mg of clarithromycin, USP and the following inactive ingredients: ammonium hydroxide, colloidal silicon dioxide, croscarmellose sodium, D&C Yellow No. 10 lake, iron-oxide black, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, propylene glycol, shellac glaze, stearic acid, talc, and titanium dioxide.
After constitution, each 5 mL of clarithromycin for oral suspension contains 125 mg or 250 mg of clarithromycin, USP. Each bottle of clarithromycin for oral suspension, contains 1250 mg (50 mL size), 2500 mg (50 and 100 mL sizes) or 5000 mg (100 mL size) of clarithromycin and the following inactive ingredients: alginic acid, aspartame*, castor oil, citric acidanhydrous, colloidal silicon dioxide, croscarmellose sodium, tutti fruitti flavor, hydroxypropyl cellulose, hypromellose, hypromellose phthalate, maltodextrin, povidone, peppermint flavor, sodium benzoate, sodium chloride, sodium citrate dihydrate, sucrose, titanium dioxide, xanthan gum.
*See PRECAUTIONSInformation for Patient/ Phenylketonurics
PHARMACOKINETICS
Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250 mg clarithromycin tablets was approximately 50%. For a single 500 mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability. Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly decrease the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, clarithromycin tablets may be given without regard to food.
In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 1 to 2 mcg/mL with a 250 mg dose administered every 12 hours and 3 to 4 mcg/mL with a 500 mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was about 3 to 4 hours with 250 mg administered every 12 hours but increased to 5 to 7 hours with 500 mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended doses of 250 mg and 500 mg administered every 8 to 12 hours. With a 250 mg every 12 hours dosing, the principal metabolite, 14-OH clarithromycin, attains a peak steady-state concentration of about 0.6 mcg/mL and has an elimination half-life of 5 to 6 hours. With a 500 mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is slightly higher (up to 1 mcg/mL), and its elimination half-life is about 7 to 9 hours. With any of these dosing regimens, the steady-state concentration of this metabolite is generally attained within 3 to 4 days.
After a 250 mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%. In comparison, after an oral dose of 250 mg (125 mg/5 mL) suspension every 12 hours, approximately 40% is excreted in urine as clarithromycin. The renal clearance of clarithromycin is, however, relatively independent of the dose size and approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with either a 250 mg or a 500 mg tablet administered every 12 hours.
Steady-state concentrations of clarithromycin and 14-OH clarithromycin observed following administration of 500 mg doses of clarithromycin every 12 hours to adult patients with HIV infection were similar to those observed in healthy volunteers. In adult HIV-infected patients taking 500 or 1000 mg doses of clarithromycin every 12 hours, steady-state clarithromycin Cmax values ranged from 2 to 4 mcg/mL and 5 to 10 mcg/mL, respectively.
The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.)
Clarithromycin and the 14-OH clarithromycin metabolite distribute readily into body tissues and fluids. There are no data available on cerebrospinal fluid penetration. Because of high intracellular concentrations, tissue concentrations are higher than serum concentrations. Examples of tissue and serum concentrations are presented below.
CONCENTRATION (after 250 mg q12h)Tissue TypeTissue (mcg/g)Serum (mcg/mL)Tonsil1.60.8Lung8.81.7When 250 mg doses of clarithromycin as clarithromycin suspension were administered to fasting healthy adult subjects, peak plasma concentrations were attained around 3 hours after dosing. Steady-state peak plasma concentrations were attained in 2 to 3 days and were approximately 2 mcg/mL for clarithromycin and 0.7 mcg/mL for 14-OH clarithromycin when 250 mg doses of the clarithromycin suspension were administered every 12 hours. Elimination half-life of clarithromycin (3 to 4 hours) and that of 14-OH clarithromycin (5 to 7 hours) were similar to those observed at steady state following administration of equivalent doses of clarithromycin tablets.
For adult patients, the bioavailability of 10 mL of the 125 mg/5 mL suspension or 10 mL of the 250 mg/5 mL suspension is similar to a 250 mg or 500 mg tablet, respectively.
In children requiring antibiotic therapy, administration of 7.5 mg/kg q12h doses of clarithromycin as the suspension generally resulted in steady-state peak plasma concentrations of 3 to 7 mcg/mL for clarithromycin and 1 to 2 mcg/mL for 14-OH clarithromycin.
In HIV-infected children taking 15 mg/kg every 12 hours, steady-state clarithromycin peak concentrations generally ranged from 6 to 15 mcg/mL.
Clarithromycin penetrates into the middle ear fluid of children with secretory otitis media.
CONCENTRATION (after 7.5 mg/kg q12h for 5 doses)AnalyteMiddle Ear Fluid (mcg/mL)Serum (mcg/mL)Clarithromycin2.51.714-OH Clarithromycin1.30.8In adults given 250 mg clarithromycin as suspension (n = 22), food appeared to decrease mean peak plasma clarithromycin concentrations from 1.2 (0.4) mcg/mL to 1 (0.4) mcg/mL and the extent of absorption from 7.2 (2.5) hrto 6.5 (3.7) hr
When children (n = 10) were administered a single oral dose of 7.5 mg/kg suspension, food increased mean peak plasma clarithromycin concentrations from 3.6 (1.5) mcg/mL to 4.6 (2.8) mcg/mL and the extent of absorption from 10 (5.5) hrto 14.2 (9.4) hr
Clarithromycin 500 mg every 8 hours was given in combination with omeprazole 40 mg daily to healthy adult males. The plasma levels of clarithromycin and 14-hydroxy-clarithromycin were increased by the concomitant administration of omeprazole. For clarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27% greater, and the mean AUC0-8 was 15% greater when clarithromycin was administered with omeprazole than when clarithromycin was administered alone. Similar results were seen for 14-hydroxy-clarithromycin, the mean Cmax was 45% greater, the mean Cmin was 57% greater, and the mean AUC0-8 was 45% greater. Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole.
Clarithromycin Tissue Concentrations 2 hours after Dose (mcg/mL)/(mcg/g)TreatmentNantrumfundusNmucusClarithromycin510.482.0120.817.6444.157.74Clarithromycin + Omeprazole519.964.7124.256.37439.29For information about other drugs indicated in combination with clarithromycin, refer to the CLINICAL PHARMACOLOGY section of their package inserts.
MICROBIOLOGY
Clarithromycin exerts its antibacterial action by binding to the 50S ribosomal subunit of susceptible microorganisms resulting in inhibition of protein synthesis.
Clarithromycin is active in vitro against a variety of aerobic and anaerobic gram-positive and gram-negative microorganisms as well as most Mycobacterium avium complex (MAC) microorganisms.
Additionally, the 14-OH clarithromycin metabolite also has clinically significant antimicrobial activity. The 14-OH clarithromycin is twice as active against Haemophilus influenzae microorganisms as the parent compound. However, for Mycobacterium avium complex (MAC) isolates the 14-OH metabolite is 4 to 7 times less active than clarithromycin. The clinical significance of this activity against Mycobacterium avium complex is unknown.
Clarithromycin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Aerobic Gram-positive Microorganisms
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobic Gram-negative Microorganisms
Haemophilus influenzae
Haemophilus parainfluenzae
Moraxella catarrhalis
Other Microorganisms
Mycoplasma pneumoniae
Chlamydia pneumoniae (TWAR)
Mycobacteria
Mycobacterium avium complex (MAC) consisting of:
Mycobacterium avium
Mycobacterium intracellulare
Beta-lactamase production should have no effect on clarithromycin activity.
NOTE: Most strains of methicillin-resistant and oxacillin-resistant staphylococci are resistant to clarithromycin.
Omeprazole/clarithromycin dual therapy; ranitidine bismuth citrate/clarithromycin dual therapy; omeprazole/clarithromycin/ amoxicillin triple therapy; and lansoprazole/clarithromycin/ amoxicillin triple therapy have been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Helicobacter
Helicobacter pylori
Pretreatment Resistance
Clarithromycin pretreatment resistance rates were 3.5% (4/113) in the omeprazole/clarithromycin dual therapy studies (M93-067, M93-100) and 9.3% (41/439) in the omeprazole/ clarithromycin/amoxicillin triple therapy studies (126, 127, M96-446). Clarithromycin pretreatment resistance was 12.6% (44/348) in the ranitidine bismuth citrate/clarithromycin b.i.d. versus t.i.d. clinical study (H2BA3001). Clarithromycin pretreatment resistance rates were 9.5% (91/960) by E-test and 11.3% (12/106) by agar dilution in the lansoprazole/clarithro-mycin/amoxicillin triple therapy clinical trials (M93-125, M93-130, M93-131, M95-392, and M95-399).
Amoxicillin pretreatment susceptible isolates (< 0.25 mcg/mL) were found in 99.3% (436/439) of the patients in the omeprazole/clarithromycin/amoxicillin clinical studies (126, 127, M96-446). Amoxicillin pretreatment minimum inhibitory concentrations (MICs) > 0.25 mcg/mL occurred in 0.7% (3/439) of the patients, all of whom were in the clarithromycin/amoxicillin study arm. Amoxicillin pretreatment susceptible isolates (< 0.25 mcg/mL) occurred in 97.8% (936/957) and 98% (98/100) of the patients in the lansoprazole/clarithromycin/amoxicillin triple- therapy clinical trials by E-test and agar dilution, respectively. Twenty-one of the 957 patients (2.2%) by E-test and 2 of 100 patients (2 %) by agar dilution had amoxicillin pretreatment MICs of > 0.25 mcg/mL. Two patients had an unconfirmed pretreatment amoxicillin minimum inhibitory concentration (MIC) of > 256 mcg/mL by E-test.
Clarithromycin Susceptibility Test Results and Clinical/Bacteriological OutcomesaClarithromycinPretreatment ResultsClarithromycin Post-Treatment ResultsH. pylori negative- eradicatedH. pylori positivenot eradicated Post-treatment susceptibility resultsSbIbRbNo MICOmeprazole 40 mg q.d./clarithromycin 500 mg t.i.d. for 14 days followed by omeprazole 20 mg q.d. for another 14 days (M93-067, M93-100)Susceptibleb108721269Intermediateb11Resistantb44Ranitidine bismuth citrate 400 mg b.i.d/clarithromycin 500 mg t.i.d. for 14 days followed by ranitidine bismuth citrate 400 mg b.i.d. for another 14 days (H2BA3001)Susceptibleb124984148Intermediateb321Resistantb171151Ranitidine bismuth citrate 400 mg b.i.d/clarithromycin 500 mg b.i.d. for 14 days followed by ranitidine bismuth citrate 400 mg b.i.d. for another 14 days (H2BA3001)Susceptibleb12510611125Intermediateb22Resistantb20119Omeprazole 20 mg b.i.d./clarithromycin 500 mg b.i.d./amoxicillin 1 g b.i.d. for 10 days (126, 127, M96-446)Susceptibleb171153738IntermediatebResistantb144163Lansoprazole 30 mg b.i.d./clarithromycin 500 mg b.i.d./amoxicillin 1 g b.i.d. for 14 days (M95-399, M93-131, M95-392)Susceptibleb1121057Intermediateb33Resistantb17674Lansoprazole 30 mg b.i.d./clarithromycin 500 mg b.i.d./amoxicillin 1 g b.i.d. for 10 days (M95-399)Susceptibleb424011IntermediatebResistantb413aIncludes only patients with pretreatment clarithromycin susceptibility tests bSusceptible (S) MIC < 0.25 mcg/mL, Intermediate (I) MIC 0.5 to 1 mcg/mL, Resistant (R) MIC > 2 mcg/mL
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
In the omeprazole/clarithromycin/amoxicillin triple therapy clinical trials, 84.9% (157/185) of the patients who had pretreatment amoxicillin susceptible MICs (< 0.25 mcg/mL) were eradicated of H. pylori and 15.1% (28/185) failed therapy. Of the 28 patients who failed triple therapy, 11 had no post-treatment susceptibility test results, and 17 had post-treatment H. pylori isolates with amoxicillin susceptible MICs. Eleven of the patients who failed triple therapy also had post-treatment H. pylori isolates with clarithromycin resistant MICs.
In the lansoprazole/clarithromycin/amoxicillin triple therapy clinical trials, 82.6% (195/236) of the patients that had pretreatment amoxicillin susceptible MICs (< 0.25 mcg/mL) were eradicated of H. pylori. Of those with pretreatment amoxicillin MICs of > 0.25 mcg/mL, three of six had the H. pylori eradicated. A total of 12.8% (22/172) of the patients failed the 10- and 14-day triple therapy regimens. Post-treatment susceptibility results were not obtained on 11 of the patients who failed therapy. Nine of the 11 patients with amoxicillin post-treatment MICs that failed the triple therapy regimen also had clarithromycin resistant H. pylori isolates.
The following in vitro data are available, but their clinical significance is unknown. Clarithromycin exhibits in vitro activity against most strains of the following microorganisms; however, the safety and effectiveness of clarithromycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic Gram-positive Microorganisms
Streptococcus agalactiae
Streptococci (Groups C, F, G)
Viridans group streptococci
Aerobic Gram-negative Microorganisms
Bordetella pertussis
Legionella pneumophila
Pasteurella multocida
Anaerobic Gram-positive Microorganisms
Clostridium perfringens
Peptococcus niger
Propionibacterium acnes
Anaerobic Gram-negative Microorganisms
Prevotella melaninogenica (formerly Bacteriodes melaninogenicus)
Susceptibility Testing Excluding Mycobacteria and Helicobacter
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of clarithromycin powder. The MIC values should be interpreted according to the following criteria:
For testingStaphylococcusspp.MIC (mcg/mL)Interpretation2Susceptible (S)4Intermediate (I)8Resistant (R)For testingStreptococcusspp.includingStreptococcus pneumoniaeaMIC (mcg/mL)Interpretation0.25Susceptible (S)0.5Intermediate (I)1Resistant (R)aThese interpretive standards are applicable only to broth microdilution susceptibility tests using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood.For testing Haemophilus spp.bMIC (mcg/mL)Interpretation8Susceptible (S)16Intermediate (I)32Resistant (R)bThese interpretive standards are applicable only to broth microdilution susceptibilty tests with Haemophilus spp. using Haemophilus Testing Medium (HTM).1Note: When testing Streptococcus spp., including Streptococcus pneumoniae, susceptibility and resistance to clarithromycin can be predicted using erythromycin.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard clarithromycin powder should provide the following MIC values:
MicroorganismMIC (mcg/mL)S. aureusATCC 292130.12 to 0.5S. pneumoniaecATCC 496190.03 to 0.12Haemophilus influenzaedATCC 492474 to 16cThis quality control range is applicable only to S. pneumoniae ATCC 49619 tested by Hyperlink microdilution procedure using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood. d This quality control range is applicable only to H. influenzae ATCC 49247 tested by Hyperlink microdilution procedure using HTM1.Diffusion Techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15 mcg clarithromycin to test the susceptibility of microorganisms to clarithromycin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 15 mcg clarithromycin disk should be interpreted according to the following criteria:
For testing Staphylococcus spp.Zone diameter (mm)Interpretation18Susceptible (S)14 to 17Intermediate (I)13Resistant (R)For testing Streptococcus spp. including Streptococcus pneumoniaeeZone diameter (mm)Interpretation21Susceptible (S)17 to 20Intermediate (I)16Resistant (R)e These zone diameter standards only apply to tests performed using Mueller-Hinton agar supplemented with 5% sheep blood incubated in 5% CO2.For testing Haemophilus spp.fZone diameter (mm)Interpretation13Susceptible (S)11 to 12Intermediate (I)10Resistant (R)fThese zone diameter standards are applicable only to tests with Haemophilus spp. using HTM2.Note: When testing Streptococcus spp., including Streptococcus pneumoniae, susceptibility and resistance to clarithromycin can be predicted using erythromycin.Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for clarithromycin.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 15 mcg clarithromycin disk should provide the following zone diameters in this laboratory test quality control strain:
MicroorganismZone diameter (mm)S. aureusATCC 2592326 to 32S. pneumoniaegATCC 4961925 to 31Haemophilus influenzaehATCC 4924711 to 17g This quality control range is applicable only to tests performed by disk diffusion using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood.h This quality control limit applies to tests conducted with Haemophilus influenzae ATCC 49247 using HTM2.In vitro Activity of Clarithromycin against Mycobacteria
Clarithromycin has demonstrated in vitro activity against Mycobacterium avium complex (MAC) microorganisms isolated from both AIDS and non-AIDS patients. While gene probe techniques may be used to distinguish M. avium species from M. intracellulare, many studies only reported results on M. avium complex (MAC) isolates.
Various in vitro methodologies employing broth or solid media at different pH's, with and without oleic acid-albumin-dextrose-catalase (OADC) have been used to determine clarithromycin MIC values for mycobacterial species. In general, MIC values decrease more than 16-fold as the pH of Middlebrook 7H12 broth media increases from 5 to 7.4. At pH 7.4, MIC values determined with Mueller-Hinton agar were 4- to 8-fold higher than those observed with Middlebrook 7H12 media. Utilization of oleic acid-albumin-dextrose-catalase (OADC) in these assays has been shown to further alter MIC values.
Clarithromycin activity against 80 MAC isolates from AIDS patients and 211 MAC isolates from non-AIDS patients was evaluated using a microdilution method with Middlebrook 7H9 broth. Results showed an MIC value of4 mcg/mL in 81% and 89% of the AIDS and non- AIDS MAC isolates, respectively. Twelve percent of the non-AIDS isolates had an MIC value0.5 mcg/mL. Clarithromycin was also shown to be active against phagocytized M. avium complex (MAC) in mouse and human macrophage cell cultures as well as in the beige mouse infection model.
Clarithromycin activity was evaluated against Mycobacterium tuberculosis microorganisms. In one study utilizing the agar dilution method with Middlebrook 7H10 media, 3 of 30 clinical isolates had an MIC of 2.5 mcg/mL. Clarithromycin inhibited all isolates at > 10 mcg/mL.
Susceptibility Testing for Mycobacterium avium Complex (MAC)
The disk diffusion and dilution techniques for susceptibility testing against gram-positive and gram-negative bacteria should not be used for determining clarithromycin MIC values against mycobacteria. In vitro susceptibility testing methods and diagnostic products currently available for determining minimum inhibitory concentration (MIC) values against Mycobacterium avium complex (MAC) organisms have not been standardized or validated. Clarithromycin MIC values will vary depending on the susceptibility testing method employed, composition and pH of the media, and the utilization of nutritional supplements. Breakpoints to determine whether clinical isolates of M. avium or M. intracellulare are susceptible or resistant to clarithromycin have not been established.
Susceptibility Test for Helicobacter pylori
The reference methodology for susceptibility testing of H. pylori is agar dilution MICs.3 One to three microliters of an inoculum equivalent to a No. 2 McFarland standard (1 x 107 to 1 x 108 CFU/mL for H. pylori) are inoculated directly onto freshly prepared antimicrobial containing Mueller-Hinton agar plates with 5% aged defibrinated sheep blood (> 2-weeks old). The agar dilution plates are incubated at 35in a microaerobic environment produced by a gas generating system suitable for Campylobacter species. After 3 days of incubation, the MICs are recorded as the lowest concentration of antimicrobial agent required to inhibit growth of the organism. The clarithromycin and amoxicillin MIC values should be interpreted according to the following criteria:
Clarithromycin MIC (mcg/mL)iInterpretation< 0.25Susceptible (S)0.5 to 1Intermediate (I)> 2Resistant (R)Amoxicillin MIC (mcg/mL) i,jInterpretation< 0.25Susceptible (S)iThese are tentative breakpoints for the agar dilution methodology, and they should not be used to interpret results obtained using alternative methods. jThere were not enough organisms with MICs > 0.25 mcg/mL to determine Hyperlink resistance breakpoint.Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard clarithromycin and amoxicillin powders should provide the following MIC values:MicroorganismsAntimicrobial AgentMIC (mcg/mL)kH. pylori ATCC 43504Clarithromycin0.015 to 0.12 mcg/mLH. pylori ATCC 43504Amoxicillin0.015 to 0.12 mcg/mLkThese are quality control ranges for the agar dilution methodology and they should not be used to control test results obtained using alternative methods.
INDICATIONS & USAGE
Clarithromycin tablets, USP and clarithromycin for oral suspension, USP are indicated for the treatment of mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions as listed below:
Adults: (clarithromycin immediate-release tablets and clarithromycin for oral suspension):
Pharyngitis/Tonsillitis due to Streptococcus pyogenes (The usual drug of choice in the treatment and prevention of streptococcal infections and the prophylaxis of rheumatic fever is penicillin administered by either the intramuscular or the oral route. Clarithromycin is generally effective in the eradication of S. pyogenes from the nasopharynx; however, data establishing the efficacy of clarithromycin in the subsequent prevention of rheumaticfever are not available at present.)
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
Acute bacterial exacerbation of chronic bronchitis due to Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
Community-Acquired Pneumonia due to Haemophilus influenzae, Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR)
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcus pyogenes (Abscesses usually require surgical drainage.)
Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacterium intracellulare
Clarithromycin tablets in combination with amoxicillin and PREVACID (lansoprazole) or PRILOSEC (omeprazole) Delayed-Release Capsules, as triple therapy, are indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or five-year history of duodenal ulcer) to eradicate H. pylori.
Clarithromycin tablets in combination with PRILOSEC (omeprazole) capsules or TRITEC (ranitidine bismuth citrate) tablets are also indicated for the treatment of patients with an active duodenal ulcer associated with H. pylori infection. However, regimens which contain clarithromycin as the single antimicrobial agent are more likely to be associated with the development of clarithromycin resistance among patients who fail therapy. Clarithromycin-containing regimens should not be used in patients with known or suspected clarithromycin resistant isolates because the efficacy of treatment is reduced in this setting.
In patients who fail therapy, susceptibility testing should be done if possible. If resistance to clarithromycin is demonstrated, a non-clarithromycin-containing therapy is recommended. (For information on development of resistance see Microbiology section.) The eradication of H. pylori has been demonstrated to reduce the risk of duodenal ulcer recurrence.
Children (clarithromycin immediate-release tablets and clarithromycin for oral suspension):
Pharyngitis/Tonsillitisdue to Streptococcus pyogenes
Community-Acquired Pneumonia due to Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR)
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
Acute otitis media due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
NOTE: For information on otitis media, see CLINICAL STUDIES: Otitis Media.
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcus pyogenes (Abscesses usually require surgical drainage.)
Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacterium intracellulare.
Prophylaxis:
Clarithromycin immediate-release tablets and clarithromycin for oral suspension are indicated for the prevention of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin and other antibacterial drugs, clarithromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
Clarithromycin is contraindicated in patients with a known hypersensitivity to clarithromycin, erythromycin, or any of the macrolide antibiotics.
Concomitant administration of clarithromycin and any of the following drugs is contraindicated: cisapride, pimozide, astemizole, terfenadine, and ergotamine or dihydroergotamine (see Drug Interactions). There have been postmarketing reports of drug interactions when clarithromycin and/or erythromycin are coadministered with cisapride, pimozide, astemizole, or terfenadine resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) most likely due to inhibition of metabolism of these drugs by erythromycin and clarithromycin. Fatalities have been reported.
For information about contraindications of other drugs indicated in combination with clarithromycin, refer to the CONTRAINDICATIONS section of their package inserts.
WARNINGS
CLARITHROMYCIN SHOULD NOT BE USED IN PREGNANT WOMEN EXCEPT IN CLINICAL CIRCUMSTANCES WHERE NO ALTERNATIVE THERAPY IS APPROPRIATE. IF PREGNANCY OCCURS WHILE TAKING THIS DRUG, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS. CLARITHROMYCIN HAS DEMONSTRATED ADVERSE EFFECTS OF PREGNANCY OUTCOME AND/OR EMBRYO-FETAL DEVELOPMENT IN MONKEYS, RATS, MICE, AND RABBITS AT DOSES THAT PRODUCED PLASMA LEVELS 2 TO 17 TIMES THE SERUM LEVELS ACHIEVED IN HUMANS TREATED AT THE MAXIMUM RECOMMENDED HUMAN DOSES. (See PRECAUTIONS - Pregnancy.)
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clarithromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C.difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
There have been postmarketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see PRECAUTIONS.)
For information about warnings of other drugs indicated in combination with clarithromycin, refer to the WARNINGS section of their package inserts.
PRECAUTIONS
General: Prescribing clarithromycinin the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Clarithromycin is principally excreted via the liver and kidney. Clarithromycin may be administered without dosage adjustment to patients with hepatic impairment and normal renal function. However, in the presence of severe renal impairment with or without coexisting hepatic impairment, decreased dosage or prolonged dosing intervals may be appropriate.
Clarithromycin in combination with ranitidine bismuth citrate therapy is not recommended in patients with creatinine clearance less than 25 mL/min. (See DOSAGE AND ADMINISTRATION.)
Clarithromycin in combination with ranitidine bismuth citrate should not be used in patients with a history of acute porphyria.
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving clarithromycin therapy.
For information about precautions of other drugs indicated in combination with clarithromycin, refer to the PRECAUTIONS section of their package inserts.
Information to Patients: Patients should be counseled that antibacterial drugs including clarithromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clarithromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clarithromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Clarithromycin may interact with some drugs; therefore patients should be advised to report to their doctor the use of any other medications.
Clarithromycin immediate-release tablets and oral suspension can be taken with or without food and can be taken with milk. Do NOT refrigerate the suspension.
Phenylketonurics: Each 125 mg/5 mL and 250 mg/5 mL of clarithromycin for oral suspension contains phenylalanine 11.2 mg/5 mL (approximately 1 teaspoonful) of suspension.
DRUG INTERACTIONS
Drug Interactions: Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range. In two studies in which theophylline was administered with clarithromycin (a theophylline sustained-release formulation was dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg q12h clarithromycin), the steady-state levels of Cmax, Cmin, and the area under the serum concentration time curve (AUC) of theophylline increased about 20%.
Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered.
When clarithromycin and terfenadine were coadministered, plasma concentrations of the active acid metabolite of terfenadine were threefold higher, on average, than the values observed when terfenadine was administered alone. The pharmacokinetics of clarithromycin and the 14-hydroxy-clarithromycin were not significantly affected by coadministration of terfenadine once clarithromycin reached steady-state conditions. Concomitant administration of clarithromycin with terfenadine is contraindicated. (See CONTRAINDICATIONS.)
Clarithromycin 500 mg every 8 hours was given in combination with omeprazole 40 mg daily to healthy adult subjects. The steady-state plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T1/2 increases of 30%, 89%, and 34%, respectively), by the concomitant administration of clarithromycin. The mean 24-hour gastric pH value was 5.2 when omeprazole was administered alone and 5.7 when coadministered with clarithromycin.
Coadministration of clarithromycin with ranitidine bismuth citrate resulted in increased plasma ranitidine concentrations (57%), increased plasma bismuth trough concentrations (48%), and increased 14-hydroxy-clarithromycin plasma concentrations (31%). These effects are clinically insignificant.
Simultaneous oral administration of clarithromycin tablets and zidovudine to HIV-infected adult patients resulted in decreased steady-state zidovudine concentrations. When 500 mg of clarithromycin were administered twice daily, steady-state zidovudine AUC was reduced by a mean of 12% (n = 4). Individual values ranged from a decrease of 34% to an increase of 14%. Based on limited data in 24 patients, when clarithromycin tablets were administered two to four hours prior to oral zidovudine, the steady-state zidovudine Cmax was increased by approximately 2-fold, whereas the AUC was unaffected.
Simultaneous administration of clarithromycin tablets and didanosine to 12 HIV-infected adult patients resulted in no statistically significant change in didanosine pharmacokinetics.
Concomitant administration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily to 21 healthy volunteers led to increases in the mean steady-state clarithromycin Cmin and AUC of 33% and 18%, respectively. Steady-state concentrations of 14-OH clarithromycin were not significantly affected by concomitant administration of fluconazole.
Concomitant administration of clarithromycin and ritonavir (n = 22) resulted in a 77% increase in clarithromycin AUC and a 100% decrease in the AUC of 14-OH clarithromycin. Clarithromycin may be administered without dosage adjustment to patients with normal renal function taking ritonavir. However, for patients with renal impairment, the following dosage adjustments should be considered. For patients with CLCR 30 to 60 mL/min, the dose of clarithromycin should be reduced by 50%. For patients with CLCR < 30 mL/min, the dose of clarithromycin should be decreased by 75%.
Spontaneous reports in the postmarketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously.
Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have also been reported in postmarketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin concentrations should be carefully monitored while patients are receiving digoxin and clarithromycin simultaneously.
Colchicine is a substrate for both CYP3A and the efflux transporter, P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered together, inhibition of Pgp and/or CYP3A by clarithromycin may lead to increased exposure to colchicine. Patients should be monitored for clinical symptoms of colhicine toxicity. (see WARNINGS.)
Erythromycin and clarithromycin are substrates and inhibitors of the 3A isoform subfamily of the cytochrome P450 enzyme system (CYP3A). Coadministration of erythromycin or clarithromycin and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both the therapeutic and adverse effects of the concomitant drug. Dosage adjustments may be considered, and when possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored closely in patients concurrently receiving clarithromycin or erythromycin.
The following are examples of some clinically significant CYP3A based drug interactions. Interactions with other drugs metabolized by the CYP3A isoform are also possible. Increased serum concentrations of carbamazepine and the active acid metabolite of terfenadine were observed in clinical trials with clarithromycin.
The following CYP3A based drug interactions have been observed with erythromycin products and/or with clarithromycin in postmarketing experience:
Antiarrhythmics: There have been postmarketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during coadministration of clarithromycin with these drugs. Serum concentrations of these medications should also be monitored.
Ergotamine/dihydroergotamine: Postmarketing reports indicate that coadministration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. Concomitant administration of clarithromycin with ergotamine or dihydroergotamine is contraindicated (see CONTRAINDICATIONS).
Triazolobenziodidiazepines (such as triazolam and alprazolam) and related benzodiazepines (such as midazolam): Erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines. There have been postmarketing reports of drug interactions and CNS effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam.
HMG-CoA Reductase Inhibitors: As with other macrolides, clarithromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (e.g., lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
Sildenafil (Viagra): Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. A similar interaction may occur with clarithromycin; reduction of sildenafil dosage should be considered. (See Viagra package insert.)
There have been spontaneous or published reports of CYP3A based interactions of erythromycin and/or clarithromycin with cyclosporine, carbamazepine, tacrolimus, alfentanil, disopyramide, rifabutin, quinidine, methylprednisolone, cilostazol, and bromocriptine.
Concomitant administration of clarithromycin with cisapride, pimozide, astemizole, or terfenadine is contraindicated. (see CONTRAINDICATIONS.)
In addition, there have been reports of interactions of erythromycin or clarithromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
The following in vitro mutagenicity tests have been conducted with clarithromycin:
Salmonella/Mammalian Microsomes Test
Bacterial Induced Mutation Frequency Test
In Vitro Chromosome Aberration Test
Rat Hepatocyte DNA Synthesis Assay
Mouse Lymphoma Assay
Mouse Dominant Lethal Study
Mouse Micronucleus Test
All tests had negative results except the In Vitro Chromosome Aberration Test which was weakly positive in one test and negative in another.
In addition, a Bacterial Reverse-Mutation Test (Ames Test) has been performed on clarithromycin metabolites with negative results.
Fertility and reproduction studies have shown that daily doses of up to 160 mg/kg/day (1.3 times the recommended maximum human dose based on mg/m2) to male and female rats caused no adverse effects on the estrous cycle, fertility, parturition, or number and viability of offspring. Plasma levels in rats after 150 mg/kg/day were 2 times the human serum levels.
In the 150 mg/kg/day monkey studies, plasma levels were 3 times the human serum levels. When given orally at 150 mg/kg/day (2.4 times the recommended maximum human dose based on mg/m2), clarithromycin was shown to produce embryonic loss in monkeys. This effect has been attributed to marked maternal toxicity of the drug at this high dose.
In rabbits, in utero fetal loss occurred at an intravenous dose of 33 mg/m2, which is 17 times less than the maximum proposed human oral daily dose of 618 mg/m2.
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of clarithromycin.
PREGNANCY
Pregnancy: Teratogenic Effects. Pregnancy Category C.
Four teratogenicity studies in rats (three with oral doses and one with intravenous doses up to 160 mg/kg/day administered during the period of major organogenesis) and two in rabbits at oral doses up to 125 mg/kg/day (approximately 2 times the recommended maximum human dose based on mg/m2) or intravenous doses of 30 mg/kg/day administered during gestation days 6 to 18 failed to demonstrate any teratogenicity from clarithromycin. Two additional oral studies in a different rat strain at similar doses and similar conditions demonstrated a low incidence of cardiovascular anomalies at doses of 150 mg/kg/day administered during gestation days 6 to 15. Plasma levels after 150 mg/kg/day were 2 times the human serum levels. Four studies in mice revealed a variable incidence of cleft palate following oral doses of 1000 mg/kg/day (2 and 4 times the recommended maximum human dose based on mg/m2, respectively) during gestation days 6 to 15. Cleft palate was also seen at 500 mg/kg/day. The 1000 mg/kg/day exposure resulted in plasma levels 17 times the human serum levels. In monkeys, an oral dose of 70 mg/kg/day (an approximate equidose of the recommended maximum human dose based on mg/m2) produced fetal growth retardation at plasma levels that were 2 times the human serum levels.
There are no adequate and well-controlled studies in pregnant women. Clarithromycin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (See WARNINGS.)
NURSING MOTHERS
Nursing Mothers: It is not known whether clarithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when clarithromycin is administered to a nursing woman. It is known that clarithromycin is excreted in the milk of lactating animals and that other drugs of this class are excreted in human milk. Preweaned rats, exposed indirectly via consumption of milk from dams treated with 150 mg/kg/day for 3 weeks, were not adversely affected, despite data indicating higher drug levels in milk than in plasma.
PEDIATRIC USE
Pediatric Use: Safety and effectiveness of clarithromycin in pediatric patients under 6 months of age have not been established. The safety of clarithromycin has not been studied in MAC patients under the age of 20 months. Neonatal and juvenile animals tolerated clarithromycin in a manner similar to adult animals. Young animals were slightly more intolerant to acute overdosage and to subtle reductions in erythrocytes, platelets, and leukocytes but were less sensitive to toxicity in the liver, kidney, thymus, and genitalia.
GERIATRIC USE
Geriatric Use: In a steady-state study in which healthy elderly subjects (age 65 to 81 years old) were given 500 mg every 12 hours, the maximum serum concentrations and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults. These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse events when compared to younger patients. Dosage adjustment should be considered in elderly patients with severe renal impairment. (see WARNINGS and PRECAUTIONS.)
ADVERSE REACTIONS
The majority of side effects observed in clinical trials were of a mild and transient nature. Fewer than 3% of adult patients without mycobacterial infections and fewer than 2% of pediatric patients without mycobacterial infections discontinued therapy because of drug-related side effects.
The most frequently reported events in adults taking clarithromycin tablets were diarrhea (3%), nausea (3%), abnormal taste (3%), dyspepsia (2%), abdominal pain/discomfort (2%), and headache (2%). In pediatric patients, the most frequently reported events were diarrhea (6%), vomiting (6%), abdominal pain (3%), rash (3%), and headache (2%). Most of these events were described as mild or moderate in severity. Of the reported adverse events, only 1% was described as severe.
In the acute exacerbation of chronic bronchitis and acute maxillary sinusitis studies overall gastrointestinal adverse events were reported by a similar proportion of patients taking either clarithromycin immediate release tablets or clarithromycin extended-release tablets; however, patients taking clarithromycin extended-release tablets reported significantly less severe gastrointestinal symptoms compared to patients taking clarithromycin immediate-release tablets. In addition, patients taking clarithromycin extended-release tablets had significantly fewer premature discontinuations for drug-related gastrointestinal or abnormal taste adverse events compared to clarithromycin immediate-release tablets.
In community-acquired pneumonia studies conducted in adults comparing clarithromycin to erythromycin base or erythromycin stearate, there were fewer adverse events involving the digestive system in clarithromycin-treated patients compared to erythromycin-treated patients (13% vs 32%; p < 0.01). Twenty percent of erythromycin-treated patients discontinued therapy due to adverse events compared to 4% of clarithromycin-treated patients.
In two U.S. studies of acute otitis media comparing clarithromycin to amoxicillin/potassium clavulanate in pediatric patients, there were fewer adverse events involving the digestive system in clarithromycin-treated patients compared to amoxicillin/potassium clavulanate-treated patients (21% vs. 40%, p < 0.001). One-third as many clarithromycin-treated patients reported diarrhea as did amoxicillin/potassium clavulanate-treated patients.
Postmarketing Experience:
Allergic reactions ranging from urticaria and mild skin eruptions to rare cases of anaphylaxis, Stevens-Johnson syndrome and toxic epidermal necrolysis have occurred. Other spontaneously reported adverse events include glossitis, stomatitis, oral moniliasis, anorexia, vomiting, pancreatitis, tongue discoloration, thrombocytopenia, leukopenia, neutropenia, and dizziness. There have been reports of tooth discoloration in patients treated with clarithromycin. Tooth discoloration is usually reversible with professional dental cleaning. There have been isolated reports of hearing loss, which is usually reversible, occurring chiefly in elderly women. Reports of alterations of the sense of smell, usually in conjunction with taste perversion or taste loss have also been reported.
Transient CNS events including anxiety, behavioral changes, confusional states, convulsions, depersonalization, disorientation, hallucinations, insomnia, manic behavior, nightmares, psychosis, tinnitus, tremor, and vertigo have been reported during postmarketing surveillance. Events usually resolve with discontinuation of the drug.
Hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, has been infrequently reported with clarithromycin. This hepatic dysfunction may be severe and is usually reversible. In very rare instances, hepatic failure with fatal outcome has been reported and generally has been associated with serious underlying diseases and/or concomitant medications.
There have been rare reports of hypoglycemia, some of which have occurred in patients taking oral hypoglycemic agents or insulin.
As with other macrolides, clarithromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes.
There have been reports of interstitial nephritis coincident with clarithromycin use.
There have been postmarketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see WARNINGS and PRECAUTIONS.)
Changes in Laboratory Values: Changes in laboratory values with possible clinical significance were as follows:
Hepatic - elevated SGPT (ALT) < 1%; SGOT (AST) < 1%; GGT < 1%; alkaline phosphatase < 1%; LDH < 1%; total bilirubin < 1%
Hematologic - decreased WBC < 1%; elevated prothrombin time 1%
Renal - elevated BUN 4%; elevated serum creatinine < 1% GGT, alkaline phosphatase, and prothrombin time data are from adult studies only.
OVERDOSAGE
Overdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea.
Adverse reactions accompanying overdosage should be treated by the prompt elimination of unabsorbed drug and supportive measures. As with other macrolides, clarithromycin serum concentrations are not expected to be appreciably affected by hemodialysis or peritoneal dialysis.
DOSAGE & ADMINISTRATION
Clarithromycin immediate-release tablets and clarithromycin for oral suspension may be given with or without food.
ADULT DOSAGE GUIDELINESClarithromycin Immediate-Release TabletsInfectionDosage (q12h)Duration (days)Pharyngitis/Tonsillitis due toS. pyogenes250mg10Acute maxillary sinusitis due toH. influenzae500 mg14M. catarrhalisS. pneumoniaeAcute exacerbation of chronic bronchitis due toH. influenzae500 mg7 to 14H. parainfluenzae500 mg7M. catarrhalis250 mg7 to 14S. pneumoniae250 mg7 to 14Community-Acquired Pneumonia due toH. influenzae250 mg7H. parainfluenzae--M. catarrhalis--S. pneumoniae250 mg7 to 14C. pneumoniae250 mg7 to 14M. pneumoniae250 mg7 to 14Uncomplicated skin and skin structure250 mg7 to14S. aureusS. pyogenesH. pylori Eradication to Reduce the Risk of Duodenal Ulcer RecurrenceTriple therapy: clarithromycin/lansoprazole/amoxicillin
The recommended adult dose is 500 mg clarithromycin, 30 mg lansoprazole, and 1 gram amoxicillin, all given twice daily (q12h) for 10 or 14 days. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.)
Triple therapy: clarithromycin/omeprazole/amoxicillin
The recommended adult dose is 500 mg clarithromycin, 20 mg omeprazole, and 1 gram amoxicillin, all given twice daily (q12h) for 10 days. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.) In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief.
Dual therapy: clarithromycin/omeprazole
The recommended adult dose is 500 mg clarithromycin given three times daily (q8h) and 40 mg omeprazole given once daily (qAM) for 14 days. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.) An additional 14 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief.
Dual therapy: clarithromycin/ranitidine bismuth citrate
The recommended adult dose is 500 mg clarithromycin given twice daily (q12h) or three times daily (q8h) and 400 mg ranitidine bismuth citrate given twice daily (q12h) for 14 days. An additional 14 days of 400 mg twice daily is recommended for ulcer healing and symptom relief. Clarithromycin and ranitidine bismuth citrate combination therapy is not recommended in patients with creatinine clearance less than 25 mL/min. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.)
Children - The usual recommended daily dosage is 15 mg/kg/day divided q12h for 10 days.
PEDIATRIC DOSAGE GUIDELINESBased on Body WeightDosing Calculated on 7.5 mg/kg q12hWeightDosekglbs(q12h)125 mg/5 mL250 mg/5 mL92062.5 mg2.5 mL q12h1.25 mL q12h1737125 mg5 mL q12h2.5 mL q12h2555187.5 mg7.5 mL q12h3.75 mL q12h3373250 mg10 mL q12h5 mL q12hClarithromycin may be administered without dosage adjustment in the presence of hepatic impairment if there is normal renal function. However, in the presence of severe renal impairment (CRCL < 30 mL/min), with or without coexisting hepatic impairment, the dose should be halved or the dosing interval doubled.
Mycobacterial infections:
Prophylaxis: The recommended dose of clarithromycin for the prevention of disseminated Mycobacterium avium disease is 500 mg b.i.d. In children, the recommended dose is 7.5 mg/kg b.i.d. up to 500 mg b.i.d. No studies of clarithromycin for MAC prophylaxis have been performed in pediatric populations and the doses recommended for prophylaxis are derived from MAC treatment studies in children. Dosing recommendations for children are in the table above.
Treatment: Clarithromycin is recommended as the primary agent for the treatment of disseminated infection due to Mycobacterium avium complex. Clarithromycin should be used in combination with other antimycobacterial drugs that have shown in vitro activity against MAC or clinical benefit in MAC treatment. (See CLINICAL STUDIES.) The recommended dose for mycobacterial infections in adults is 500 mg b.i.d. In children, the recommended dose is 7.5 mg/kg b.i.d. up to 500 mg b.i.d. Dosing recommendations for children are in the table above.
Clarithromycin therapy should continue for life if clinical and mycobacterial improvements are observed.
Constituting Instructions
The table below indicates the volume of water to be added when constituting.
Total volumeafter constitutionClarithromycin concentration after constitutionAmount of water to be added*50 mL125 mg/5 mL32 mL100 mL125 mg/5 mL64 mL50 mL250 mg/5 mL32 mL100 mL250 mg/5 mL64 mL*see instructions below.Add half the volume of water to the bottle and shake vigorously. Add the remainder of water to the bottle and shake.
Shake well before using. Oversize bottle provides shake space. Keep tightly closed. Do not refrigerate. After mixing, store at 20 - 25C (68 - 77F) (see USP Controlled Room Temperature) and use within 14 days.
HOW SUPPLIED
Clarithromycin Tablets, USP 250 mg and 500 mg are light yellow, capsule shaped, biconvex, film coated tablets. They are supplied as follows:
250 mg tablets: are printed in black ink withRX 725'on one side and plain on the other side. They are supplied as follows:
NDC 63304-725-82 Bottles of 12
NDC 63304-725-05 Bottles of 500
NDC 63304-725-77 Blister unit-dose of 100 (10 x 10)
500 mg tablets: are printed in black ink withRX 726'on one side and plain on the other side. They are supplied as follows:
NDC 63304-726-82 Bottles of 12
NDC 63304-726-05 Bottles of 500
NDC 63304-726-77 Blister unit-dose of 100 (10 x 10)
Store at 20 - 25C (68 - 77F) (see USP Controlled Room Temperature) in a well closed container.
Clarithromycin for Oral Suspension, USP is a white to off-white granular powder forming white to off-white suspension on constitution with water. The resulting suspension has a sweet taste and fruity flavor. They are supplied as follows:
125 mg/5 mL
NDC 63304-821-03 50 mL Bottles
NDC 63304-821-04100 mL Bottles
250 mg/5 mL
NDC 63304-822-03 50 mL Bottles
NDC 63304-822-04100 mL Bottles
Store clarithromycin for oral suspension at 20 - 25C (68 - 77F) (see USP Controlled Room Temperature) in a well closed container. Do not refrigerate clarithromycin for oral suspension
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
Clarithromycin is rapidly and well-absorbed with dose-linear kinetics, low protein binding, and a high volume of distribution. Plasma half-life ranged from 1 to 6 hours and was species dependent. High tissue concentrations were achieved, but negligible accumulation was observed. Fecal clearance predominated. Hepatotoxicity occurred in all species tested (i.e., in rats and monkeys at doses 2 times greater than and in dogs at doses comparable to the maximum human daily dose, based on mg/m2). Renal tubular degeneration (calculated on a mg/m2 basis) occurred in rats at doses 2 times, in monkeys at doses 8 times, and in dogs at doses 12 times greater than the maximum human daily dose. Testicular atrophy (on a mg/m2 basis) occurred in rats at doses 7 times, in dogs at doses 3 times, and in monkeys at doses 8 times greater than the maximum human daily dose. Corneal opacity (on a mg/m2 basis) occurred in dogs at doses 12 times and in monkeys at doses 8 times greater than the maximum human daily dose. Lymphoid depletion (on a mg/m2 basis) occurred in dogs at doses 3 times greater than and in monkeys at doses 2 times greater than the maximum human daily dose. These adverse events were absent during clinical trials.
REFERENCES
1. National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically - Fourth Edition. Approved Standard NCCLS Document M7-A4, Vol. 17, No. 2, NCCLS, Wayne, PA, January 1997.
2. National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests - Sixth Edition. Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1, NCCLS, Wayne, PA, January, 1997.
3. National Committee for Clinical Laboratory Standards. Summary Minutes, Subcommittee on Antimicrobial Susceptibility Testing, Tampa, FL. January 11-13, 1998.
4. Chaisson RE, et al. Clarithromycin and Ethambutol with or without Clofazimine for the Treatment of Bacteremic Mycobacterium avium Complex Disease in Patients with HIV Infection. AIDS. 1997;11:311-317.
5. Kemper CA, et al. Treatment of Mycobacterium avium Complex Bacteremia in AIDS with a Four-Drug Oral Regimen. Ann Intern Med. 1992;116:466-472.
Mycobacterial Infections
Prophylaxis:
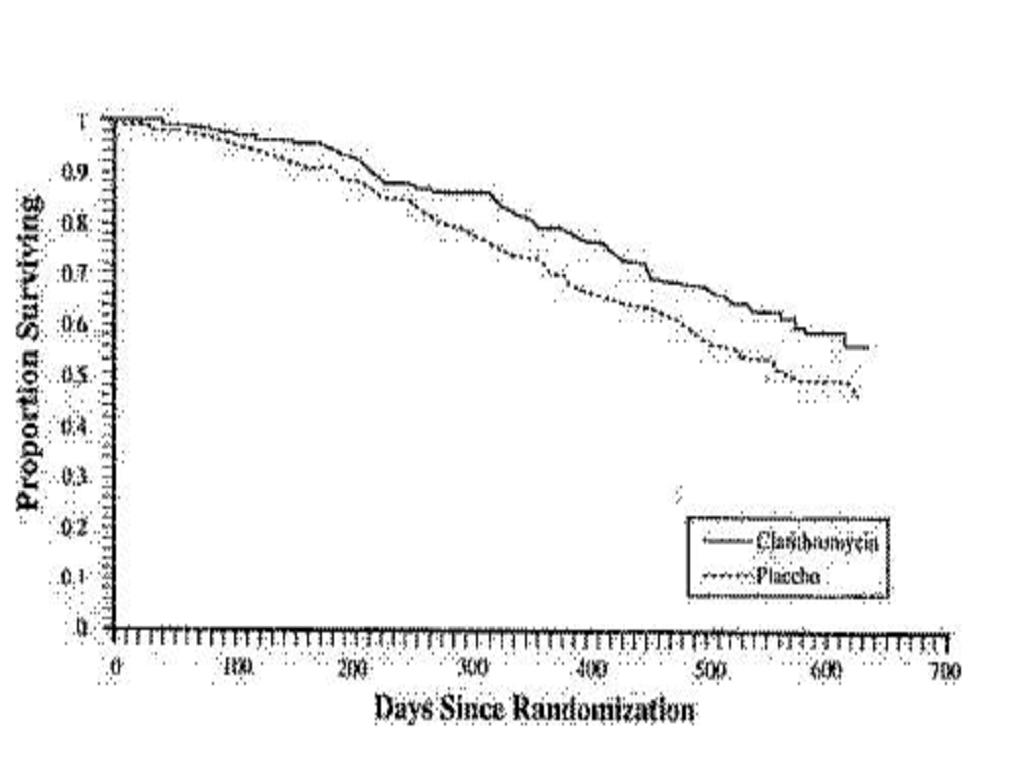
A randomized, double-blind study (561) compared clarithromycin 500 mg b.i.d. to placebo in patients with CDC-defined AIDS and CD4 counts < 100 cells/This study accrued 682 patients from November 1992 to January 1994, with a median CD4 cell count at study entry of 30 cells/Median duration of clarithromycin was 10.6 months vs. 8.2 months for placebo. More patients in the placebo arm than the clarithromycin arm discontinued prematurely from the study (75.6% and 67.4%, respectively). However, if premature discontinuations due to MAC or death are excluded, approximately equal percentages of patients on each arm (54.8% on clarithromycin and 52.5% on placebo) discontinued study drug early for other reasons. The study was designed to evaluate the following endpoints:
MAC bacteremia:
In patients randomized to clarithromycin, the risk of MAC bacteremia was reduced by 69% compared to placebo. The difference between groups was statistically significant (p < 0.001). On an intent-to-treat basis, the one-year cumulative incidence of MAC bacteremia was 5 % for patients randomized to clarithromycin and 19.4% for patients randomized to placebo. While only 19 of the 341 patients randomized to clarithromycin developed MAC, 11 of these cases were resistant to clarithromycin. The patients with resistant MAC bacteremia had a median baseline CD4 count of 10 cells/mm3 (range 2 to 25 cells/mm3). Information regarding the clinical course and response to treatment of the patients with resistant MAC bacteremia is limited. The 8 patients who received clarithromycin and developed susceptible MAC bacteremia had a median baseline CD4count of 25 cells/mm3 (range 10 to 80 cells/mm3). Comparatively, 53 of the 341 placebo patients developed MAC; none of these isolates were resistant to clarithromycin. The median baseline CD4 count was 15 cells/mm3 (range 2 to 130 cells/mm3) for placebo patients that developed MAC.
Survival:
A statistically significant survival benefit was observed.
| CLARITHROMYCIN
clarithromycin tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |