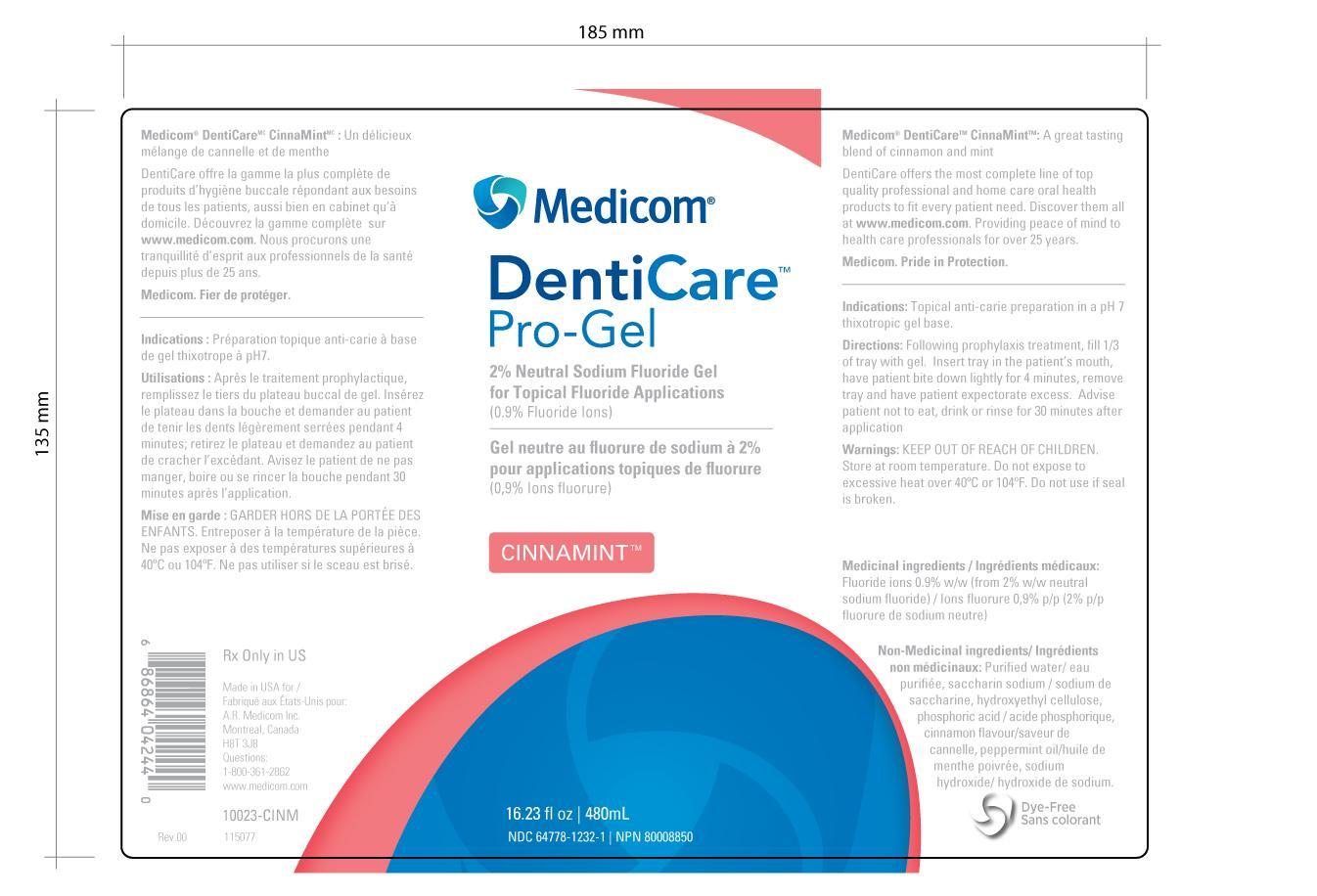
DENTICARE PRO-GEL- sodium fluoride gel
AMD Medicom Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
10023-CINM (NDC 1232) CinnaMint Gel
DentiCare Pro-Gel 2% Neutral Sodium Fluoride
Keep out of reach of children. Store at room temperature. Do not expose to excessive heat over 40C or 104F. Do not use if the seal is broken.
Medicom
DentiCare Pro-Gel
2% Neutral Sodium Fluoride Gel for Topical Fluoride Application
Cinnamint
10023-CINM
Purified water, saccharin sodium hydroxyethyl cellulose, phosphoric acid, cinnamon flavor, peppermint oil, sodium hydroxide
| DENTICARE PRO-GEL
sodium fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - AMD Medicom Inc (256880576) |
| Registrant - AMD Medicom Inc (256880576) |
Revised: 4/2019
Document Id: 860f61dd-204a-4c79-e053-2a91aa0afb04
Set id: ea96ae34-d526-4cf6-aa71-dd71d49347e5
Version: 2
Effective Time: 20190408
AMD Medicom Inc