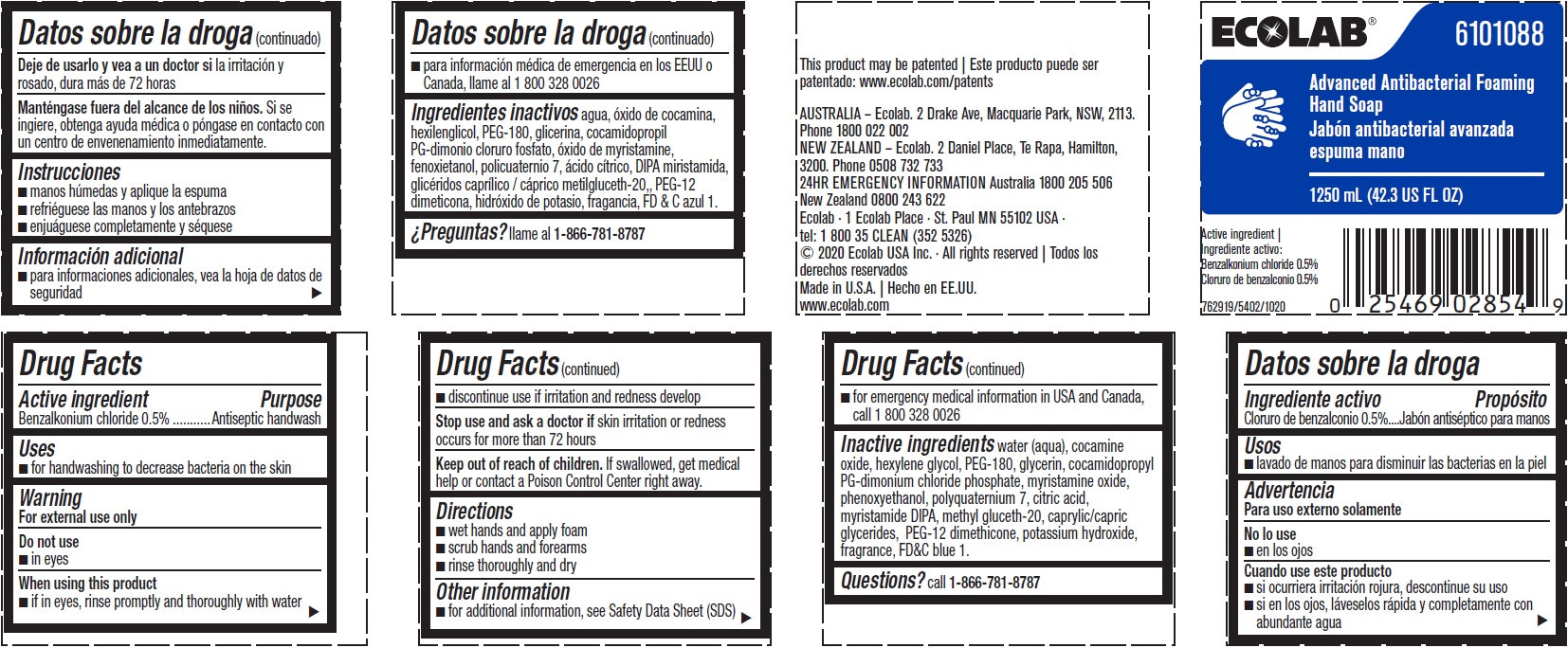
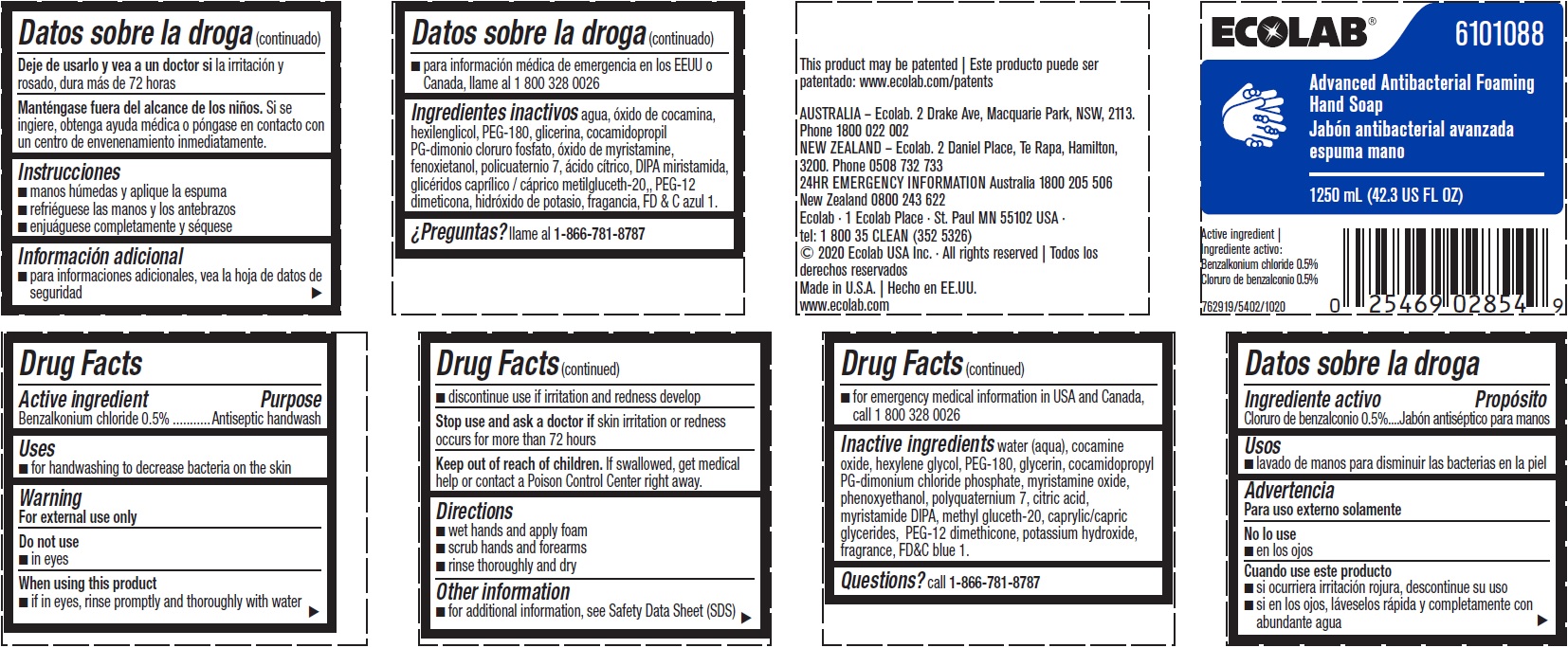
Label: ADVANCED ANTIBACTERIAL FOAMING HAND SOAP- benzalkonium chloride solution
-
NDC Code(s):
47593-543-41,
47593-543-44,
47593-543-59,
47593-543-60, view more47593-543-63
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warning
- Directions
- Other information
-
INACTIVE INGREDIENT
Inactive ingredients water (aqua), cocamine oxide, hexylene glycol, PEG-180, glycerin, cocamidopropyl PG-dimonium chloride phosphate, myristamine oxide, phenoxyethanol, polyquaternium 7, citric acid, myristamide DIPA, methyl gluceth-20, caprylic/capric glycerides, PEG-12 dimethicone, potassium hydroxide, fragrance, FD&C blue 1
- QUESTIONS
- Representative Label and Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ADVANCED ANTIBACTERIAL FOAMING HAND SOAP
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-543 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) METHYL GLUCETH-20 (UNII: J3QD0LD11P) MYRISTIC DIISOPROPANOLAMIDE (UNII: 17DN142CTK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 900000 MW) (UNII: B70CUU14M9) PHENOXYETHANOL (UNII: HIE492ZZ3T) MYRISTAMINE OXIDE (UNII: J086PM3RRT) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) HEXYLENE GLYCOL (UNII: KEH0A3F75J) WATER (UNII: 059QF0KO0R) COCAMINE OXIDE (UNII: QWA2IZI6FI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-543-60 1600 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/12/2015 2 NDC:47593-543-63 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/12/2015 3 NDC:47593-543-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/12/2015 4 NDC:47593-543-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/12/2015 5 NDC:47593-543-44 1134000 mL in 1 CONTAINER; Type 0: Not a Combination Product 08/12/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/12/2015 Labeler - Ecolab Inc. (006154611)