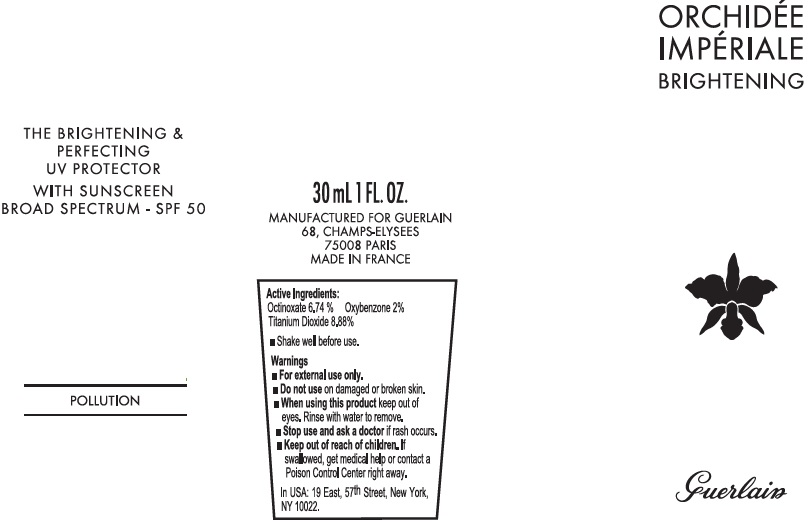
ORCHIDEE IMPERIALE BRIGHTENING EXCEPTIONAL COMPLETE CARE THE BRIGHTENING AND PERFECTING UV PROTECTOR WITH SUNSCREEN BROAD SPECTRUM SPF 50 POLLUTION- octinoxate, oxybenzone, titanium dioxide cream
Guerlain
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ORCHIDEE IMPERIALE BRIGHTENING - EXCEPTIONAL COMPLETE CARE - THE BRIGHTENING & PERFECTING UV PROTECTOR WITH SUNSCREEN - BROAD SPECTRUM - SPF 50 - POLLUTION
Directions
For sunscreen use:
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every two hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months: Ask a doctor.
Question or comments?
In USA: 19 East, 57th Street, New York, NY 10022
855 454 6242 Monday through Friday 9am to 5pm EST.
Inactive ingredients
AQUA (WATER) ● C12-15 ALKYL BENZOATE ● BUTYLENE GLYCOL ● ISODODECANE ● GLYCERIN ● CI 77891 (TITANIUM DIOXIDE) ● SILICA ● VINYL DIMETHICONE/ METHICONE SILSESQUIOXANE CROSSPOLYMER ● CETYL PEG/PPG-10/1 DIMETHICONE ● ALUMINA ● ALUMINUM STEARATE ● POLYHYDROXYSTEARIC ACID ● MAGNESIUM SULFATE ● GASTRODIA ELATA ROOT EXTRACT ● PHALAENOPSIS AMABILIS EXTRACT ● DENDROBIUM NOBILE EXTRACT ● PHENOXYETHANOL ● PARFUM (FRAGRANCE) ● SORBITAN SESQUIOLEATE ● CI 77492 (IRON OXIDES) ● CAPRYLYL GLYCOL ● SYNTHETIC FLUORPHLOGOPITE ● DISTEARDIMONIUM HECTORITE ● SODIUM MYRISTOYL GLUTAMATE ● CI 77491 (IRON OXIDES) ● PROPYLENE CARBONATE ● ALUMINUM HYDROXIDE ● LINALOOL ● MORINGA PTERYGOSPERMA SEED EXTRACT ● CI 77499 (IRON OXIDES) ● BHT ● DISODIUM PHOSPHATE ● CITRONELLOL ● CITRIC ACID ● GERANIOL ● TROPAEOLUM MAJUS EXTRACT ● TIN OXIDE ● ALPHA-ISOMETHYL IONONE ● LIMONENE ● STEARIC ACID ● ETHYLHEXYLGLYCERIN ● PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE ● HEXAPEPTIDE-2 ● TOCOPHEROL
| ORCHIDEE IMPERIALE BRIGHTENING EXCEPTIONAL COMPLETE CARE THE BRIGHTENING AND PERFECTING UV PROTECTOR WITH SUNSCREEN BROAD SPECTRUM SPF 50 POLLUTION
octinoxate, oxybenzone, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guerlain (266623064) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guerlain | 266623064 | manufacture(49817-8001) | |