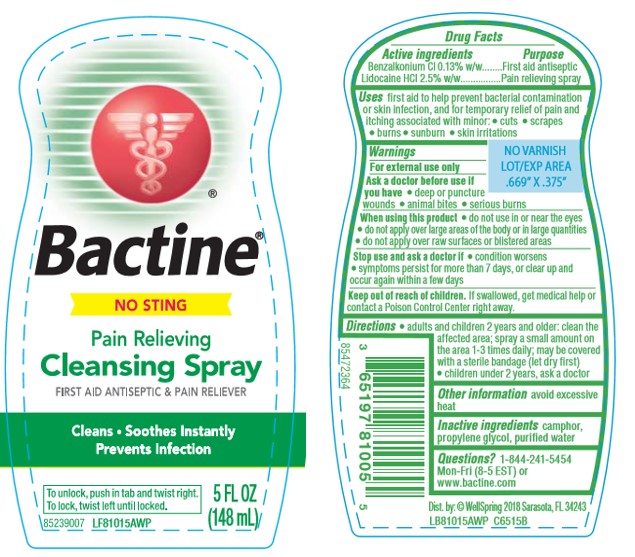
BACTINE PAIN RELIEVING- benzalkonium chloride and lidocaine hydrochloride liquid
WellSpring Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bactine® Pain Relieving Cleansing Spray
Uses
first aid to help prevent bacterial contamination or skin infection, and for temporary relief of pain and itching associated with minor:
- cuts
- scrapes
- burns
- sunburn
- skin irritations
Warnings
For external use only
When using this product
- do not use in or near the eyes
- do not apply over large areas of the body or in large quantities
- do not apply over raw surfaces or blistered areas
| BACTINE PAIN RELIEVING
benzalkonium chloride and lidocaine hydrochloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - WellSpring Pharmaceutical Corporation (110999054) |
Revised: 1/2020
Document Id: 87acf15c-63a6-4d7d-a132-111074b0a5fd
Set id: ea1ad81e-b658-4ba8-b78e-6acbfb9a8622
Version: 9
Effective Time: 20200117
WellSpring Pharmaceutical Corporation