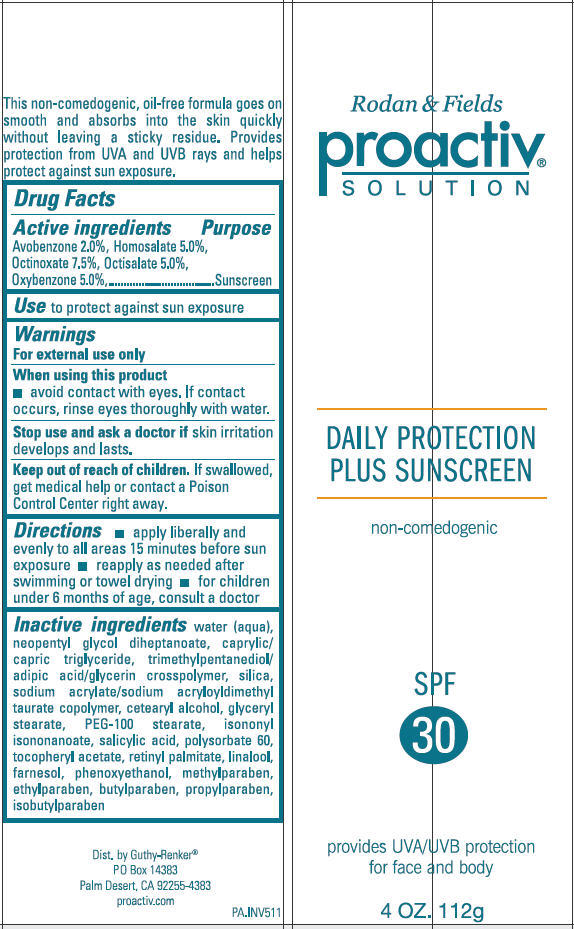
PROACTIV SOLUTION DAILY PROTECTION PLUS SUNSCREEN SPF 30- avobenzone, homosalate, octinoxate, octisalate, and oxybenzone cream
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Solution Daily Protection Plus Sunscreen SPF 30
Active Ingredient
Avobenzone 2.0%, Homosalate 5.0%, Octinoxate 7.5%, Octisalate 5.0%, Oxybenzone 5.0%
Warnings
For external use only
Directions
- Apply liberally and evenly to all areas 15 minutes before sun exposure
- Reapply as needed after swimming or towel drying.
- For children under 6 months of age, consult a doctor.
Inactive Ingredients
water, neopentyl glycol diheptanoate, caprylic/capric triglyceride, trimethylpentanediol/adipic acid/glycerin crosspolymer, silica, sodium acrylate/sodium acryloyldimethyl taurate copolymer, cetearyl alcohol, glyceryl stearate, PEG-100 stearate, isononyl isononanoate, salicylic acid, polysorbate 60, tocopheryl acetate, retinyl palmitate, linalool, farnesol, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, isobutylparaben
| PROACTIV SOLUTION
DAILY PROTECTION PLUS SUNSCREEN SPF 30
avobenzone, homosalate, octinoxate, octisalate, and oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Registrant - THE PROACTIV COMPANY LLC (080216357) |