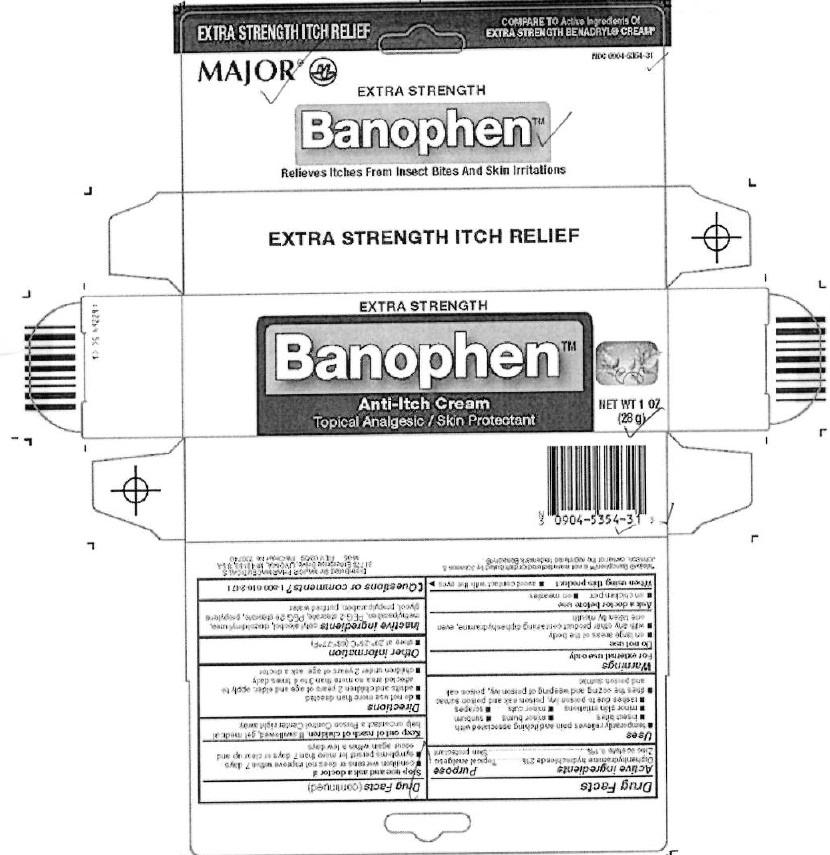
BANOPHEN- diphenhydramine hydrochloride, zinc acetate cream
REMEDYREPACK INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Major Pharmaceuticals Banophen™ Drug Facts
INDICATIONS & USAGE
- temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak and poison sumac
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
WARNINGS
For external use only
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
- on chicken pox
- on measles
- avoid contact with the eyes
- condition worsens or does not improve within 7 days
- symptoms persist for more than 7 days or clear up and occur again within a few days
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION
- do not use more than directed
- adults and children 2 years of age and older: apply to affected area no more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
INACTIVE INGREDIENT
cetyl alcohol, diazolidinyl urea, methylparaben, PEG-2 stearate, PEG-20 stearate, propylene glycol, propylparaben, purified water
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Banophen
GENERIC: Diphenhydramine Hydrochloride, Zinc Acetate
DOSAGE: CREAM
ADMINSTRATION: TOPICAL
NDC: 61786-194-06
ACTIVE INGREDIENT(S):
- DIPHENHYDRAMINE HYDROCHLORIDE 2g in 100g
- ZINC ACETATE 0.1g in 100g
INACTIVE INGREDIENT(S):
- CETYL ALCOHOL
PACKAGING: 28 g in 1 TUBE
OUTER PACKAGING: 1 TUBE in 1 CARTON

| BANOPHEN
diphenhydramine hydrochloride, zinc acetate cream |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |