Label: INTERSOL- platelet additive 3 solution
- NDC Code(s): 0942-9601-12
- Packager: Fenwal, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
Rx only
For use with the AMICUS Separator only.
Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat. Definition of "Controlled Room Temperature":
"A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15°C and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses. Provided the mean kinetic temperature remains in the allowed range, transient spikes up to 40°C are permitted as long as they do not exceed 24 hours ... The mean kinetic temperature is a calculated value that may be used as an isothermal storage temperature that simulates the non isothermal effects of storage temperature variations."
Reference: United States Pharmacopeia, General Notices. United States Pharmacopeial Convention, Inc. 12601 Twinbrook Parkway, Rockville, MD.
Dispose of waste in appropriate biohazard container or according to local regulatory requirements.Indications and Usage:
InterSol solution is an isotonic solution designed to replace a proportion of the plasma used in the storage of AMICUS™-derived leukoreduced apheresis platelets under standard blood banking conditions. There is no direct therapeutic effect to be expected from the formulation. The solution should never be infused directly into a patient.InterSol platelets are leukocyte-reduced apheresis platelet concentrates that are stored in a mix of 65% InterSol and 35% plasma, nominal. InterSol platelets prepared within the range of Table 1 may be stored for up to 5 days at 20-24°C, with continuous agitation.
-
DOSAGE & ADMINISTRATION
Dosage and Administration:
InterSol solution may only be used with the AMICUS Separator System using an AMICUS Apheresis Kit with a Platelet Additive Solution (PAS) Connector. For full instructions on the use of InterSol solution with the AMICUS separator, see the AMICUS Operator’s Manual.Prior to use of InterSol solution, check the plastic container for leaks by squeezing the bag firmly. If leaks are found, discard solution.
To connect the InterSol solution to the AMICUS Apheresis Kit:
1) Close the clamp on the PAS Connector line.
2) Remove the protective cap from the membrane port on the InterSol solution container.
3) Insert the spike on the PAS Connector line into the membrane port on the InterSol solution container until firmly seated.
4) For the addition of InterSol solution to the platelet product, follow the instructions provided in the AMICUS Separator Operator’s Manual. -
DOSAGE FORMS & STRENGTHS
Dosage Forms and Strengths:
InterSol solution is provided as a 500 mL sterile and non-pyrogenic solution in a non-PVC plastic container with a sterile and non-pyrogenic fluid path. Each 100 mL contains 305 mg Dibasic Sodium Phosphate, Anhydrous, USP; 93 mg Monobasic Sodium Phosphate, Monohydrate, USP; 318 mg Sodium Citrate, Dihydrate, USP; 442 mg Sodium Acetate, Trihydrate, USP; 452 mg Sodium Chloride, USP; Water for Injection, USP quantity sufficient. -
CONTRAINDICATIONS
Contraindications:
InterSol solution is added to AMICUS-derived leukoreduced platelet concentrates after the apheresis procedure is complete. It is not for direct intravenous infusion. There are no known contraindications associated with the use of InterSol solution for the preparation of InterSol platelets. -
WARNINGS AND PRECAUTIONS
Warnings and Precautions:
- •
- InterSol solution is NOT FOR DIRECT INTRAVENOUS INFUSION.
- •
- Do not use if particulate matter is present or if the solution is cloudy.
- •
- Do not use if the container is damaged, leaking or if there is any visible sign of deterioration.
- •
- Do not vent.
- •
- Do not reuse. Discard unused or partially used InterSol solution.
- •
- Protect from sharp objects.
- •
- Verify that the InterSol solution has been securely attached to the PAS Connector line to avoid disconnection and leaks.
-
ADVERSE REACTIONS
Adverse Reactions:
InterSol solution is added to AMICUS-derived leukoreduced platelet concentrates after the apheresis procedure is complete. It is not for direct intravenous infusion. InterSol solution is not expected to cause adverse events other than those normally associated with platelet transfusion. - DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DESCRIPTION
Description:
InterSol solution is an isotonic solution designed to replace a proportion of the plasma used in the storage of platelets. The solution contains constituents that are naturally occurring components present in many cellular systems: sodium acetate as a nutrient, sodium citrate to prevent platelet clumping and activation, sodium phosphate for buffering and sodium chloride for osmolarity. InterSol solution does not have a pharmacological effect in vivo, but rather acts to provide the appropriate environment and nutrients in lieu of a portion of the plasma normally used for the storage of platelets. - CLINICAL PHARMACOLOGY
-
CLINICAL STUDIES
Clinical Studies:
In Vitro Biochemical and Functional Evaluations
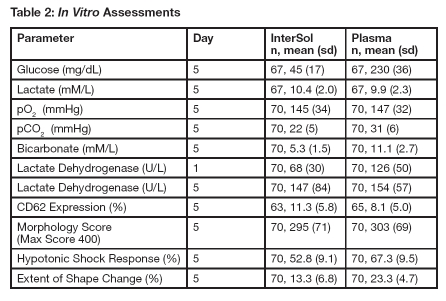
InterSol platelet concentrates (n=70) prepared using the AMICUS separator and stored for 5 days showed a median and mean pH value on Day 5 of 7.2 and 7.2 ± 0.1 (range: 6.9-7.5), respectively, with a lower non-parametric 95%/95% tolerance limit of 6.9.Supplemental in vitro assessments of InterSol platelets and AMICUS-derived leukoreduced platelets stored in 100% plasma are presented in Table 2.
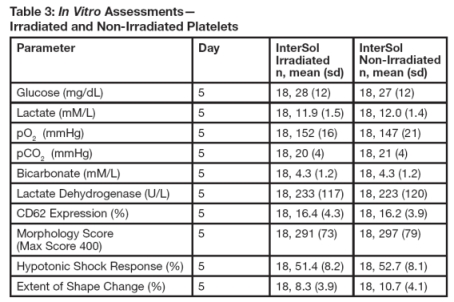
InterSol platelets irradiated (n=18) at either 2500 or 2800 cGray (dependent on site procedures) were compared to non-irradiated InterSol platelets. At the end of storage on Day 5 the platelet yields of the irradiated products were concentrated between 2.5 x 10^11 to 3.5 x 10^11 and included one product with a yield less than2.5 x 10^11 and two products with yields greater than 3.5 x 10^11. In vitro testing is not predictive of in vivo performance, which was not evaluated. Summary statistics are presented in Table 3.
In Vivo Recovery and Survival in Healthy Subjects
In vivo evaluation of InterSol platelets at Day 5 (n=33) compared to a fresh platelet control resulted in a mean percent recovery of 46.4 ± 11.9 percent and 58.0 ± 10.7 percent and mean survival of 5.7 ± 1.4 days and 8.0 ± 1.4 days, respectively. The in vivo data collected were used to calculate the upper limit of a two-sided 95% confidence interval of the mean percent recovery of the algebraic expression (0.66 x Fresh – 5 Day), and of the mean percent survival (days) of the algebraic expression (0.58 x Fresh – 5 Day). The upper bound of the two-sided 95% confidence intervals for recovery and survival on Day 5 was –4.6 and –0.6 respectively and met the requirement of less than 0.
Post-Market Transfusion-Related Adverse Events (AE) Study
An open label, non-randomized, retrospective medical record review study was performed in 6 centers to demonstrate that the overall rate of transfusion-related AEs in patients receiving InterSol Platelet transfusions was not more than double the rate in patients receiving apheresis platelets stored in 100% plasma (Plasma Platelets). The study categorized adverse transfusion reactions according to the definitions outlined by the Biovigilance Component of the National Healthcare Safety Network (NHSN) System. All sites that participated were required to have systems in place to observe transfusion-related AEs and to record when no transfusion-related AE was observed. An independent Clinical Events Committee (CEC) blinded to platelet type and study site adjudicated the reported events.
Patients were prescribed platelet transfusions per each site’s standard practice. The type of platelet unit the patient received was based on the site’s inventory at the time the transfusion was ordered. Site personnel observed the platelet transfusion recipient following their standard procedures. Signs and symptoms of a potential transfusion-related reaction were noted using existing reporting systems. A total of 14,005 transfusions from 6 study sites were included in the final analysis. A total of 4,160 InterSol Platelet transfusions were given to a total of 1,444 patients, and 9,845 Plasma Platelet transfusions were given to 2,202 patients. There were 165 CEC-adjudicated adverse reactions reported. Of those, 23 events were associated with InterSol Platelets and 142 events were associated with Plasma Platelets.
Overall, 1.13% of all transfusions resulted in an AE. The percentage of InterSol Platelet transfusions which led to AEs was 0.55%, while 1.37% of Plasma Platelet transfusions resulted in AEs. The 97.5% upper confidence limit for the relative risk of transfusion-related AE associated with InterSol Platelets relative to Plasma Platelets was 0.66, indicating that the study objective of ruling out a doubling of transfusion-related AEs for InterSol vs. Plasma Platelets was met.
The majority of the 165 CEC-adjudicated reactions were Allergic (n=93) or Febrile Non-Hemolytic Transfusions Reactions (FNHTR, n=56), at 0.66% and 0.40% of total transfusions, respectively. There were allergic reactions associated with 0.29% of InterSol Platelet transfusions and 0.82% of Plasma Platelet transfusions. FNHTR events were associated with 0.17% of InterSol Platelet transfusions and 0.50% of Plasma Platelet transfusions.
No InterSol Platelet transfusion was associated with more than one reaction. Seven (7) Plasma Platelet transfusions (0.07% of total transfusions) were associated with two separate adverse reactions each. Two (2) InterSol Platelet and 5 Plasma Platelet adverse reactions were classified as severe. All other reactions were classified as non-severe. All adverse reactions were reported with an outcome of “Minor or No Sequelae”.
No adverse safety trends were identified after review of all the AEs by the independent Clinical Events Committee.
-
HOW SUPPLIED
How Supplied/Storage and Handling:
500 mL sterile solution in a non-PVC plastic container with a sterile, non-pyrogenic fluid path. The InterSol solution container is supplied in a vented plastic overwrap covering that serves as a dust cover for the container. The dust cover is not a sterility barrier and is not an element that defines the expiration date of the InterSol product.

© 2016 Fresenius Kabi AG. All rights reserved.
Fresenius Kabi AG
61346 Bad Homburg / Germany
Tel.: +49 (0) 61 72 / 686-0
www.fresenius-kabi.com
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Code X6B7850
NDC No. 0942-9601-12
Rx only
500mL
(en) InterSol Solution Platelet Additive Solution 3
For use with the AMICUS separator only. Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
Discard unused portion. Each 100 mL contains 305 mg Dibasic Sodium Phosphate, Anhydrous, USP; 93 mg Monobasic Sodium Phosphate, Monohydrate, USP; 318 mg Sodium Citrate, Dihydrate, USP; 442 mg Sodium Acetate, Trihydrate, USP; 452 mg Sodium Chloride, USP; Water for Injection, USP quantity sufficient.
NOT FOR DIRECT INTRAVENOUS INFUSION.
(es) Solución InterSol Solución aditiva para plaquetas 3
Para ser usada exclusivamente con el separador AMICUS. Almacenar a temperatura ambiente controlada. Proteger de la congelación. Evitar el calor excesivo. Deseche la porción no utilizada. Cada 100 mL contienen 305 mg de fosfato de sodio dibásico, anhidro, USP; 93 mg de fosfato de sodio monobásico, monohidratado, USP; 318 mg de citrato de sodio, dihidratado, USP; 442 mg de acetato de sodio, trihidratado, USP; 452 mg de cloruro de sodio, USP; agua para inyección, USP, en cantidad suficiente.
NO PARA INFUSIÓN INTRAVENOSA DIRECTA.
Manufacturer
Fresenius Kabi AG
61346 Bad Homburg / Germany
Tel.: +49 (0) 61 72 / 686-0
Manufacturing facility / Manufactured by
Fenwal International Inc.
Road 357 K.M. 0.8
Maricao, Puerto Rico 00606
Made in US
Importado y distribuido por:
Fresenius Kabi Colombia S.A.S.
Avenida Carrera 7° No. 155c-20 Piso 40
Bogotá, Colombia
Phone: +57 (1) 7 56 04 04
Reg. San. No.
INVIMA 2010DM-0006825
47-17-12-086 REV:A
-
INGREDIENTS AND APPEARANCE
INTERSOL
platelet additive 3 solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0942-9601 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) Sodium Chloride 452 mg in 100 mL SODIUM ACETATE (UNII: 4550K0SC9B) (ACETATE ION - UNII:569DQM74SC) SODIUM ACETATE 442 mg in 100 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 318 mg in 100 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM 305 mg in 100 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 93 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0942-9601-12 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN080041 10/25/2012 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-9601)