VENTOLIN HFA- albuterol sulfate aerosol, metered
Clinical Solutions Wholesale
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VENTOLIN HFA safely and effectively. See full prescribing information for VENTOLIN HFA.
VENTOLIN HFA (albuterol sulfate) Inhalation Aerosol Initial U.S. Approval: 1981 INDICATIONS AND USAGEVENTOLIN HFA is a beta 2-adrenergic agonist indicated for: (1)
DOSAGE AND ADMINISTRATIONFOR ORAL INHALATION ONLY. (2)
DOSAGE FORMS AND STRENGTHSInhalation aerosol: 108 mcg albuterol sulfate (90 mcg albuterol base) from mouthpiece per actuation. Supplied in 18-g canister containing 200 actuations and 8-g canister containing 60 actuations. (3) CONTRAINDICATIONSHypersensitivity to albuterol sulfate or any of the ingredients of VENTOLIN HFA. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥3%) are throat irritation, viral respiratory infections, upper respiratory inflammation, cough, and musculoskeletal pain. (6) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 8/2016 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
Administer VENTOLIN HFA by oral inhalation only. Shake VENTOLIN HFA well before each spray.
2.1 Bronchospasm
For treatment of acute episodes of bronchospasm or prevention of symptoms associated with bronchospasm, the usual dosage for adults and children is 2 inhalations repeated every 4 to 6 hours; in some patients, 1 inhalation every 4 hours may be sufficient. More frequent administration or a larger number of inhalations is not recommended.
2.2 Exercise-Induced Bronchospasm
The usual dosage for adults and children 4 years of age and older is 2 inhalations 15 to 30 minutes before exercise.
2.3 Administration Information
Priming: Priming VENTOLIN HFA is essential to ensure appropriate albuterol content in each actuation. Prime VENTOLIN HFA before using for the first time, when the inhaler has not been used for more than 2 weeks, or when the inhaler has been dropped. To prime VENTOLIN HFA, release 4 sprays into the air away from the face, shaking well before each spray.
Cleaning: To ensure proper dosing and to prevent actuator orifice blockage, wash the actuator with warm water and let it air-dry completely at least once a week.
Dose Counter: VENTOLIN HFA has a dose counter attached to the canister that starts at 204 or 64 and counts down each time a spray is released [see Dosage Forms and Strengths (3)]. When the counter reads 020, the patient should contact the pharmacist for a refill of medication or consult the physician to determine whether a prescription refill is needed.
VENTOLIN HFA comes in a moisture-protective foil pouch, which should be removed prior to use. Discard VENTOLIN HFA when the counter reads 000 or 12 months after removal from the moisture-protective foil pouch, whichever comes first [see Dosage Forms and Strengths (3)].
See Patient Information tear-off leaflet for instructions on how to prime and clean the inhaler to ensure proper dosing and to prevent actuator orifice blockage.
3 DOSAGE FORMS AND STRENGTHS
VENTOLIN HFA is an inhalation aerosol. Each actuation contains 108 mcg albuterol sulfate (90 mcg albuterol base) from the mouthpiece. VENTOLIN HFA is supplied as an 18-g pressurized aluminum canister with dose counter fitted with a blue plastic actuator and a blue strapcap; this canister contains 200 actuations. VENTOLIN HFA is also supplied as an 8-g pressurized aluminum canister with dose counter fitted with a blue plastic actuator and a blue strapcap; this canister contains 60 actuations.
4 CONTRAINDICATIONS
VENTOLIN HFA is contraindicated in patients with a history of hypersensitivity to albuterol or any other components of VENTOLIN HFA. Rare cases of hypersensitivity reactions, including urticaria, angioedema, and rash have been reported after the use of albuterol sulfate.
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm
Inhaled albuterol sulfate can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs, VENTOLIN HFA should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister.
5.2 Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of VENTOLIN HFA than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
5.3 Use of Anti-Inflammatory Agents
The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids, to the therapeutic regimen.
5.4 Cardiovascular Effects
VENTOLIN HFA, like all other beta 2-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients such as changes in pulse rate or blood pressure. If such effects occur, VENTOLIN HFA may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical relevance of these findings is unknown. Therefore, VENTOLIN HFA, like all other sympathomimetic amines, should be used with caution in patients with underlying cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.5 Do Not Exceed Recommended Dose
Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs in patients with asthma. The exact cause of death is unknown, but cardiac arrest following an unexpected development of a severe acute asthmatic crisis and subsequent hypoxia is suspected.
5.6 Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of albuterol sulfate inhalation aerosol, as demonstrated by cases of urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. Discontinue VENTOLIN HFA if immediate hypersensitivity reactions occur.
5.7 Coexisting Conditions
VENTOLIN HFA, like other sympathomimetic amines, should be used with caution in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus and in patients who are unusually responsive to sympathomimetic amines. Large doses of intravenous albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
6 ADVERSE REACTIONS
Use of VENTOLIN HFA may be associated with the following:
- Paradoxical bronchospasm [see Warnings and Precautions (5.1)]
- Cardiovascular effects [see Warnings and Precautions (5.4)]
- Immediate hypersensitivity reactions [see Warnings and Precautions (5.6)]
- Hypokalemia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
The safety data described below reflects exposure to VENTOLIN HFA in 248 patients treated with VENTOLIN HFA in 3 placebo-controlled clinical trials of 2 to 12 weeks’ duration. The data from adults and adolescents is based upon 2 clinical trials in which 202 patients with asthma 12 years of age and older were treated with VENTOLIN HFA 2 inhalations 4 times daily for 12 weeks’ duration. The adult/adolescent population was 92 female, 110 male and 163 white, 19 black, 18 Hispanic, 2 other. The data from pediatric patients are based upon 1 clinical trial in which 46 patients with asthma 4 to 11 years of age were treated with VENTOLIN HFA 2 inhalations 4 times daily for 2 weeks’ duration. The population was 21 female, 25 male and 25 white, 17 black, 3 Hispanic, 1 other.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults and Adolescents 12 Years of Age and Older: The two 12-week, randomized, double-blind studies in 610 adolescent and adult patients with asthma that compared VENTOLIN HFA, a CFC 11/12-propelled albuterol inhaler, and an HFA-134a placebo inhaler. Overall, the incidence and nature of the adverse reactions reported for VENTOLIN HFA and a CFC 11/12-propelled albuterol inhaler were comparable. Table 1 lists the incidence of all adverse reactions (whether considered by the investigator to be related or unrelated to drug) from these studies that occurred at a rate of 3% or greater in the group treated with VENTOLIN HFA and more frequently in the group treated with VENTOLIN HFA than in the HFA-134a placebo inhaler group.
|
Adverse Reaction |
Percent of Patients |
||
|
VENTOLIN HFA (n = 202) % |
CFC 11/12-Propelled Albuterol Inhaler (n = 207) % |
Placebo HFA-134a (n = 201) % |
|
|
Ear, nose, and throat | |||
|
Throat irritation |
10 |
6 |
7 |
|
Upper respiratory inflammation |
5 |
5 |
2 |
|
Lower respiratory | |||
|
Viral respiratory infections |
7 |
4 |
4 |
|
Cough |
5 |
2 |
2 |
|
Musculoskeletal | |||
|
Musculoskeletal pain |
5 |
5 |
4 |
|
aThis table includes all adverse reactions (whether considered by the investigator to be drug-related or unrelated to drug) that occurred at an incidence rate of at least 3.0% in the group treated with VENTOLIN HFA and more frequently in the group treated with VENTOLIN HFA than in the HFA-134a placebo inhaler group. |
|||
Adverse reactions reported by less than 3% of the adolescent and adult patients receiving VENTOLIN HFA and by a greater proportion of patients receiving VENTOLIN HFA than receiving HFA-134a placebo inhaler and that have the potential to be related to VENTOLIN HFA include diarrhea, laryngitis, oropharyngeal edema, cough, lung disorders, tachycardia, and extrasystoles. Palpitation and dizziness have also been observed with VENTOLIN HFA.
Pediatric Patients: Results from the 2-week pediatric clinical study in patients with asthma 4 to 11 years of age showed that this pediatric population had an adverse reaction profile similar to that of the adolescent and adult populations.
Three studies have been conducted to evaluate the safety and efficacy of VENTOLIN HFA in patients between birth and 4 years of age. The results of these studies did not establish the efficacy of VENTOLIN HFA in this age-group [see Pediatric Use (8.4)]. Since the efficacy of VENTOLIN HFA has not been demonstrated in children between birth and 48 months of age, the safety of VENTOLIN HFA in this age-group cannot be established. However, the safety profile observed in the pediatric population under 4 years of age was comparable to that observed in the older pediatric patients and in adolescents and adults. Where adverse reaction incidence rates were greater in patients under 4 years of age compared with older patients, the higher incidence rates were noted in all treatment arms, including placebo. These adverse reactions included upper respiratory tract infection, nasopharyngitis, pyrexia, and tachycardia.
6.2 Postmarketing Experience
In addition to the adverse reactions listed in section 6.1, the following adverse reactions have been identified during postapproval use of VENTOLIN HFA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cases of paradoxical bronchospasm, hoarseness, arrhythmias (including atrial fibrillation, supraventricular tachycardia), and hypersensitivity reactions (including urticaria, angioedema, rash) have been reported after the use of VENTOLIN HFA.
In addition, albuterol, like other sympathomimetic agents, can cause adverse reactions such as hypokalemia, hypertension, peripheral vasodilatation, angina, tremor, central nervous system stimulation, hyperactivity, sleeplessness, headache, muscle cramps, drying or irritation of the oropharynx, and metabolic acidosis.
7 DRUG INTERACTIONS
Other short-acting sympathomimetic aerosol bronchodilators should not be used concomitantly with albuterol. If additional adrenergic drugs are to be administered by any route, they should be used with caution to avoid deleterious cardiovascular effects.
7.1 Beta-Blockers
Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as VENTOLIN HFA, but may produce severe bronchospasm in patients with asthma. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers should be considered, although they should be administered with caution.
7.2 Diuretics
The ECG changes and/or hypokalemia that may result from the administration of nonpotassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical relevance of these effects is not known, caution is advised in the coadministration of beta-agonists with nonpotassium-sparing diuretics. Consider monitoring potassium levels.
7.3 Digoxin
Mean decreases of 16% to 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical relevance of these findings for patients with obstructive airway disease who are receiving inhaled albuterol and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and albuterol.
7.4 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
VENTOLIN HFA should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of albuterol on the vascular system may be potentiated. Consider alternative therapy in patients taking MAOs or tricyclic antidepressants.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C.
There are no adequate and well-controlled studies of VENTOLIN HFA or albuterol sulfate in pregnant women. During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. No consistent pattern of defects can be discerned, and a relationship between albuterol use and congenital anomalies has not been established. Animal reproduction studies in mice and rabbits revealed evidence of teratogenicity. VENTOLIN HFA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In a mouse reproduction study, subcutaneously administered albuterol sulfate produced cleft palate formation in 5 of 111 (4.5%) fetuses at exposures approximately equal to the maximum recommended human dose (MRHD) for adults on a mg/m 2 basis and in 10 of 108 (9.3%) fetuses at approximately 8 times the MRHD. Similar effects were not observed at approximately one eleventh of the MRHD. Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with isoproterenol (positive control).
In a rabbit reproduction study, orally administered albuterol sulfate produced cranioschisis in 7 of 19 fetuses (37%) at approximately 680 times the MRHD.
In another rabbit study, an albuterol sulfate/HFA-134a formulation administered by inhalation produced enlargement of the frontal portion of the fetal fontanelles at approximately one third of the MRHD [see Animal Toxicology and/or Pharmacology (13.2)].
8.2 Labor and Delivery
Because of the potential for beta-agonist interference with uterine contractility, use of VENTOLIN HFA for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
8.3 Nursing Mothers
Plasma levels of albuterol sulfate and HFA-134a after inhaled therapeutic doses are very low in humans, but it is not known whether the components of VENTOLIN HFA are excreted in human milk. Because of the potential for tumorigenicity shown for albuterol in animal studies and lack of experience with the use of VENTOLIN HFA by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution should be exercised when VENTOLIN HFA is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of VENTOLIN HFA in children 4 years of age and older has been established based upon two 12-week clinical trials in patients 12 years of age and older with asthma and one 2-week clinical trial in patients 4 to 11 years of age with asthma [see Clinical Studies (14.1), Adverse Reactions (6.1)]. The safety and effectiveness of VENTOLIN HFA in children under 4 years of age has not been established. Three studies have been conducted to evaluate the safety and efficacy of VENTOLIN HFA in patients under 4 years of age and the findings are described below.
Two 4-week randomized, double-blind, placebo-controlled studies were conducted in 163 pediatric patients from birth to 48 months of age with symptoms of bronchospasm associated with obstructive airway disease (presenting symptoms included: wheeze, cough, dyspnea, or chest tightness). VENTOLIN HFA or placebo HFA was delivered with either an AeroChamber Plus ® Valved Holding Chamber or an Optichamber ® Valved Holding Chamber with mask 3 times daily. In one study, VENTOLIN HFA 90 mcg (N = 26), VENTOLIN HFA 180 mcg (N = 25), and placebo HFA (N = 26) were administered to children between 24 and 48 months of age. In the second study, VENTOLIN HFA 90 mcg (N = 29), VENTOLIN HFA 180 mcg (N = 29), and placebo HFA (N = 28) were administered to children between birth and 24 months of age. Over the 4-week treatment period, there were no treatment differences in asthma symptom scores between the groups receiving VENTOLIN HFA 90 mcg, VENTOLIN HFA 180 mcg, and placebo in either study.
In a third study, VENTOLIN HFA was evaluated in 87 pediatric patients younger than 24 months of age for the treatment of acute wheezing. VENTOLIN HFA was delivered with an AeroChamber Plus Valved Holding Chamber in this study. There were no significant differences in asthma symptom scores and mean change from baseline in an asthma symptom score between VENTOLIN HFA 180 mcg and VENTOLIN HFA 360 mcg.
In vitro dose characterization studies were performed to evaluate the delivery of VENTOLIN HFA via holding chambers with facemasks. The studies were conducted with 2 different holding chambers with facemasks (small and medium size). The in vitro study data when simulated to patients suggest that the dose of VENTOLIN HFA presented for inhalation via a valved holding chamber with facemask will be comparable to the dose delivered in adults without a spacer and facemask per kilogram of body weight (Table 2). However, clinical studies in children under 4 years of age described above suggest that either the optimal dose of VENTOLIN HFA has not been defined in this age-group or VENTOLIN HFA is not effective in this age-group. The safety and effectiveness of VENTOLIN HFA administered with or without a spacer device in children under 4 years of age has not been demonstrated.
|
Age |
Facemask |
Flow Rate (L/min) |
Holding Time (seconds) |
Mean Medication Delivery Through AeroChamber Plus (mcg/actuation) |
Body Weight 50 th Percentile (kg) a |
Medication Delivered per Actuation (mcg/kg) b |
|
6 to 12 Months |
Small |
4.9 |
0 2 5 10 |
18.2 19.8 13.8 15.4 |
7.5-9.9 |
1.8-2.4 2.0-2.6 1.4-1.8 1.6-2.1 |
|
2 to 5 Years |
Small |
8.0 |
0 2 5 10 |
17.8 16.0 16.3 18.3 |
12.3-18.0 |
1.0-1.4 0.9-1.3 0.9-1.3 1.0-1.5 |
|
2 to 5 Years |
Medium |
8.0 |
0 2 5 10 |
21.1 15.3 18.3 18.2 |
12.3-18.0 |
1.2-1.7 0.8-1.2 1.0-1.5 1.0-1.5 |
|
>5 Years |
Medium |
12.0 |
0 2 5 10 |
26.8 20.9 19.6 20.3 |
18.0 |
1.5 1.2 1.1 1.1 |
|
aCenters for Disease Control growth charts, developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000). Ranges correspond to the average of the 50 th percentile weight for boys and girls at the ages indicated. bA single inhalation of VENTOLIN HFA in a 70-kg adult without use of a valved holding chamber and facemask delivers approximately 90 mcg, or 1.3 mcg/kg. |
||||||
8.5 Geriatric Use
Clinical studies of VENTOLIN HFA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, sleeplessness. Hypokalemia and metabolic acidosis may also occur.
As with all sympathomimetic aerosol medications, cardiac arrest and even death may be associated with abuse of VENTOLIN HFA. Treatment consists of discontinuation of VENTOLIN HFA together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of VENTOLIN HFA.
The oral median lethal dose of albuterol sulfate in mice is greater than 2,000 mg/kg (approximately 6,800 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 3,200 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In mature rats, the subcutaneous median lethal dose of albuterol sulfate is approximately 450 mg/kg (approximately 3,000 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 1,400 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In young rats, the subcutaneous median lethal dose is approximately 2,000 mg/kg (approximately 14,000 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 6,400 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). The inhalation median lethal dose has not been determined in animals.
11 DESCRIPTION
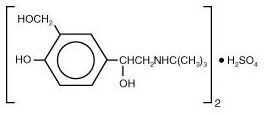
The active component of VENTOLIN HFA is albuterol sulfate, USP, the racemic form of albuterol and a relatively selective beta 2-adrenergic bronchodilator. Albuterol sulfate has the chemical name α 1-[( tert-butylamino)methyl]-4-hydroxy- m-xylene-α, α′-diol sulfate (2:1)(salt) and the following chemical structure:
Albuterol sulfate is a white crystalline powder with a molecular weight of 576.7, and the empirical formula is (C 13H 21NO 3) 2•H 2SO 4. It is soluble in water and slightly soluble in ethanol.
The World Health Organization recommended name for albuterol base is salbutamol.
VENTOLIN HFA is a pressurized metered-dose aerosol unit fitted with a counter. VENTOLIN HFA is intended for oral inhalation only. Each unit contains a microcrystalline suspension of albuterol sulfate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
Priming VENTOLIN HFA is essential to ensure appropriate albuterol content in each actuation. To prime the inhaler, release 4 sprays into the air away from the face, shaking well before each spray. The inhaler should be primed before using it for the first time, when it has not been used for more than 2 weeks, or when it has been dropped.
After priming, each actuation of the inhaler delivers 120 mcg of albuterol sulfate, USP in 75 mg of suspension from the valve and 108 mcg of albuterol sulfate, USP from the mouthpiece (equivalent to 90 mcg of albuterol base from the mouthpiece).
Each 18-g canister provides 200 inhalations. Each 8-g canister provides 60 inhalations.
This product does not contain chlorofluorocarbons (CFCs) as the propellant.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta 2-adrenergic receptors compared with isoproterenol. While it is recognized that beta 2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta 2-receptors in the human heart existing in a concentration between 10% and 50% of cardiac beta-adrenergic receptors. The precise function of these receptors has not been established [see Warnings and Precautions (5.4)].
Activation of beta 2-adrenergic receptors on airway smooth muscle leads to the activation of adenylcyclase and to an increase in the intracellular concentration of cyclic-3′,5′-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP leads to the activation of protein kinase A, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation. Albuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway.
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes [see Warnings and Precautions (5.4)].
12.2 Pharmacokinetics
The systemic levels of albuterol are low after inhalation of recommended doses. A study conducted in 12 healthy male and female subjects using a higher dose (1,080 mcg of albuterol base) showed that mean peak plasma concentrations of approximately 3 ng/mL occurred after dosing when albuterol was delivered using propellant HFA-134a. The mean time to peak concentrations (T max) was delayed after administration of VENTOLIN HFA (T max = 0.42 hours) as compared to CFC-propelled albuterol inhaler (T max = 0.17 hours). Apparent terminal plasma half-life of albuterol is approximately 4.6 hours. No further pharmacokinetic studies for VENTOLIN HFA were conducted in neonates, children, or elderly subjects.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a dose-related increase in the incidence of benign leiomyomas of the mesovarium at and above dietary doses of 2.0 mg/kg (approximately 14 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 6 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In another study this effect was blocked by the coadministration of propranolol, a non-selective beta-adrenergic antagonist. In an 18-month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 500 mg/kg (approximately 1,700 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 800 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In a 22-month study in Golden hamsters, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 50 mg/kg (approximately 225 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 110 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis).
Albuterol sulfate was not mutagenic in the Ames test or a mutation test in yeast. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleus assay.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses of albuterol sulfate up to 50 mg/kg (approximately 340 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
13.2 Animal Toxicology and/or Pharmacology
Preclinical: Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5.0% of the plasma concentrations. In structures outside the blood-brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical relevance of these findings is unknown.
Propellant HFA-134a is devoid of pharmacological activity except at very high doses in animals (380 to 1,300 times the maximum human exposure based on comparisons of AUC values), primarily producing ataxia, tremors, dyspnea, or salivation. These are similar to effects produced by the structurally related CFCs, which have been used extensively in metered-dose inhalers.
In animals and humans, propellant HFA-134a was found to be rapidly absorbed and rapidly eliminated, with an elimination half-life of 3 to 27 minutes in animals and 5 to 7 minutes in humans. Time to maximum plasma concentration (T max) and mean residence time are both extremely short, leading to a transient appearance of HFA-134a in the blood with no evidence of accumulation.
Reproductive Toxicology Studies: A study in CD-1 mice given albuterol sulfate subcutaneously showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis) and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg (approximately 8 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). The drug did not induce cleft palate formation at a dose of 0.025 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with 2.5 mg/kg of isoproterenol (positive control).
A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 fetuses (37%) when albuterol sulfate was administered orally at a 50 mg/kg dose (approximately 680 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
In an inhalation reproduction study in New Zealand white rabbits, albuterol sulfate/HFA-134a formulation exhibited enlargement of the frontal portion of the fetal fontanelles at and above inhalation doses of 0.0193 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
A study in which pregnant rats were dosed with radiolabeled albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
14 CLINICAL STUDIES
14.1 Bronchospasm Associated With Asthma
Adult and Adolescent Patients 12 Years of Age and Older: The efficacy of VENTOLIN HFA was evaluated in two 12-week, randomized, double-blind, placebo controlled trials in patients 12 years of age and older with mild to moderate asthma. These trials included a total of 610 patients (323 males, 287 females). In each trial, patients received 2 inhalations of VENTOLIN HFA, CFC 11/12-propelled albuterol, or HFA-134a placebo 4 times daily for 12 weeks’ duration. Patients taking the HFA-134a placebo inhaler also took VENTOLIN HFA for asthma symptom relief on an as-needed basis. Some patients who participated in these clinical trials were using concomitant inhaled steroid therapy. Efficacy was assessed by serial forced expiratory volume in 1 second (FEV 1). In each of these trials, 2 inhalations of VENTOLIN HFA produced significantly greater improvement in FEV 1 over the pretreatment value than placebo. Results from the 2 clinical trials are described below.
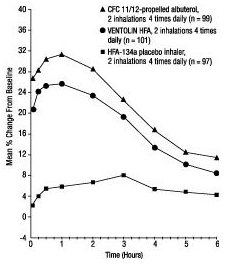
In a 12-week, randomized, double-blind study, VENTOLIN HFA (101 patients) was compared to CFC 11/12-propelled albuterol (99 patients) and an HFA-134a placebo inhaler (97 patients) in adolescent and adult patients 12 to 76 years of age with mild to moderate asthma. Serial FEV 1 measurements [shown below as percent change from test-day baseline at Day 1 (n = 297) and at Week 12 (n = 249)] demonstrated that 2 inhalations of VENTOLIN HFA produced significantly greater improvement in FEV 1 over the pretreatment value than placebo.
FEV 1 as Percent Change From Predose in a Large, 12-Week Clinical Trial
Day 1
Week 12
In the responder population (≥15% increase in FEV 1 within 30 minutes postdose) treated with VENTOLIN HFA, the mean time to onset of a 15% increase in FEV 1 over the pretreatment value was 5.4 minutes, and the mean time to peak effect was 56 minutes. The mean duration of effect as measured by a 15% increase in FEV 1 over the pretreatment value was approximately 4 hours. In some patients, duration of effect was as long as 6 hours.
The second 12-week randomized, double-blind study was conducted to evaluate the efficacy and safety of switching patients from CFC 11/12-propelled albuterol to VENTOLIN HFA. During the 3-week run-in phase of the study, all patients received CFC 11/12-propelled albuterol. During the double-blind treatment phase, VENTOLIN HFA (91 patients) was compared to CFC 11/12-propelled albuterol (100 patients) and an HFA-134a placebo inhaler (95 patients) in adolescent and adult patients with mild to moderate asthma. Serial FEV 1 measurements demonstrated that 2 inhalations of VENTOLIN HFA produced significantly greater improvement in pulmonary function than placebo. The switching from CFC 11/12-propelled albuterol inhaler to VENTOLIN HFA did not reveal any clinically significant changes in the efficacy profile.
In the 2 adult studies, the efficacy results from VENTOLIN HFA were significantly greater than placebo and were clinically comparable to those achieved with CFC 11/12-propelled albuterol, although small numerical differences in mean FEV 1 response and other measures were observed. Physicians should recognize that individual responses to beta-adrenergic agonists administered via different propellants may vary and that equivalent responses in individual patients should not be assumed.
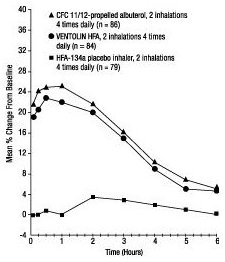
Pediatric Patients 4 Years of Age: The efficacy of VENTOLIN HFA was evaluated in one 2-week, randomized, double-blind, placebo-controlled trial in 135 pediatric patients 4 to 11 years of age with mild to moderate asthma. In this trial, patients received VENTOLIN HFA, CFC 11/12-propelled albuterol, or HFA-134a placebo. Serial pulmonary function measurements demonstrated that 2 inhalations of VENTOLIN HFA produced significantly greater improvement in pulmonary function than placebo and that there were no significant differences between the groups treated with VENTOLIN HFA and CFC 11/12-propelled albuterol. In the responder population treated with VENTOLIN HFA, the mean time to onset of a 15% increase in peak expiratory flow rate (PEFR) over the pretreatment value was 7.8 minutes, and the mean time to peak effect was approximately 90 minutes. The mean duration of effect as measured by a 15% increase in PEFR over the pretreatment value was greater than 3 hours. In some patients, duration of effect was as long as 6 hours.
14.2 Exercise-Induced Bronchospasm
One controlled clinical study in adult patients with asthma (N = 24) demonstrated that 2 inhalations of VENTOLIN HFA taken approximately 30 minutes prior to exercise significantly prevented exercise-induced bronchospasm (as measured by maximum percentage fall in FEV 1 following exercise) compared to an HFA-134a placebo inhaler. In addition, VENTOLIN HFA was shown to be clinically comparable to a CFC 11/12-propelled albuterol inhaler for this indication.
16 HOW SUPPLIED/STORAGE AND HANDLING
VENTOLIN HFA (albuterol sulfate) Inhalation Aerosol is supplied in the following packs as a pressurized aluminum canister fitted with a counter with a blue plastic actuator and a blue strapcap packaged within a moisture-protective foil pouch that also contains a desiccant:
NDC 0173-0682-20 18-g canister containing 200 actuations
NDC 0173-0682-21 8-g canister containing 60 actuations
NDC 0173-0682-24 8-g institutional pack canister containing 60 actuations
Before using, VENTOLIN HFA should be removed from the moisture-protective foil pouch. The pouch and desiccant should be discarded. VENTOLIN HFA should be discarded 12 months after removal from the pouch.
Priming VENTOLIN HFA is essential to ensure appropriate albuterol content in each actuation. To prime the inhaler, release 4 sprays into the air away from the face, shaking well before each spray. The inhaler should be primed before using it for the first time, when the inhaler has not been used for more than 2 weeks, or when it has been dropped.
After priming, each actuation delivers 120 mcg of albuterol sulfate, USP in 75 mg of suspension from the valve and 108 mcg of albuterol sulfate, USP from the mouthpiece (equivalent to 90 mcg of albuterol base from the mouthpiece).
To ensure proper dosing and to prevent actuator orifice blockage, wash the actuator with warm water and let it air-dry completely at least once a week [see the Patient Information tear‑off leaflet].
The blue actuator supplied with VENTOLIN HFA should not be used with any other product canisters, and actuators from other products should not be used with a VENTOLIN HFA canister.
VENTOLIN HFA has a counter attached to the canister. The counter starts at 204 or 64 and counts down each time a spray is released. The correct amount of medication in each inhalation cannot be assured after the counter reads 000, even though the canister is not completely empty and will continue to operate. VENTOLIN HFA should be discarded when the counter reads 000 or 12 months after removal from the moisture-protective foil pouch, whichever comes first. Never immerse the canister in water to determine the amount of drug remaining in the canister.
Keep out of reach of children. Avoid spraying in eyes.
Contents Under Pressure: Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator.
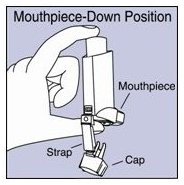
Store between 15° and 25°C (59° and 77°F). Store the inhaler with the mouthpiece down. For best results, the inhaler should be at room temperature before use. SHAKE WELL BEFORE EACH SPRAY.
VENTOLIN HFA does not contain chlorofluorocarbons (CFCs) as the propellant.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
17.1 Frequency of Use
The action of VENTOLIN HFA should last up to 4 to 6 hours. VENTOLIN HFA should not be used more frequently than recommended. Do not increase the dose or frequency of doses of VENTOLIN HFA without consulting the physician. If patients find that treatment with VENTOLIN HFA becomes less effective for symptomatic relief, symptoms become worse, and/or they need to use the product more frequently than usual, they should seek medical attention immediately.
17.2 Priming and Cleaning
Priming: Patients should be instructed that priming VENTOLIN HFA is essential to ensure appropriate albuterol content in each actuation. Patients should prime VENTOLIN HFA before using for the first time, when the inhaler has not been used for more than 2 weeks, or when the inhaler has been dropped. To prime VENTOLIN HFA, patients should release 4 sprays into the air away from the face, shaking well before each spray.
Cleaning: To ensure proper dosing and to prevent actuator orifice blockage, patients should be instructed to wash the actuator and dry thoroughly at least once a week. Patients should be informed that detailed cleaning instructions are included in the Patient Information leaflet.
17.3 Dose Counter
Patients should be informed that VENTOLIN HFA has a dose counter that starts at 204 or 64 and counts down each time a spray is released. Patients should be informed to discard VENTOLIN HFA when the counter reads 000 or 12 months after removal from the moisture-protective foil pouch, whichever comes first. When the counter reads 020, the patient should contact the pharmacist for a refill of medication or consult the physician to determine whether a prescription refill is needed. Patients should never try to alter the numbers or remove the counter from the metal canister. Patients should never immerse the canister in water to determine the amount of drug remaining in the canister.
17.4 Paradoxical Bronchospasm
Patients should be informed that VENTOLIN HFA can produce paradoxical bronchospasm. If paradoxical bronchospasm occurs, patients should discontinue VENTOLIN HFA.
17.5 Concomitant Drug Use
While patients are using VENTOLIN HFA, other inhaled drugs and asthma medications should be taken only as directed by the physician.
17.6 Common Adverse Effects
Common adverse effects of treatment with inhaled albuterol include palpitations, chest pain, rapid heart rate, tremor, and nervousness.
17.7 Pregnancy
Patients who are pregnant or nursing should contact their physicians about the use of VENTOLIN HFA.
VENTOLIN is a registered trademark of GlaxoSmithKline.
AeroChamber Plus is a registered trademark of Monaghan Medical Inc.
OptiChamber is a registered trademark of Respironics Inc.
- GlaxoSmithKline
- Research Triangle Park, NC 27709
©2012, GlaxoSmithKline. All rights reserved.
VNT:8PI
- PHARMACIST—DETACH HERE AND GIVE LEAFLET TO PATIENT
Patient Information
VENTOLIN ® HFA (vent′ o-lin)
(albuterol sulfate)
Inhalation Aerosol
Read this Patient Information before you start using VENTOLIN HFA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is VENTOLIN HFA?
VENTOLIN HFA is a prescription medicine used in people 4 years of age and older to:
- treat or prevent bronchospasm in people who have reversible obstructive airway disease
- prevent exercise-induced bronchospasm
It is not known if VENTOLIN HFA is safe and effective in children under 4 years of age.
Who should not use VENTOLIN HFA?
Do not use VENTOLIN HFA if you are allergic to albuterol sulfate or any of the ingredients in VENTOLIN HFA. See the end of this Patient Information leaflet for a complete list of ingredients in VENTOLIN HFA.
What should I tell my healthcare provider before I use VENTOLIN HFA?
Before you use VENTOLIN HFA, tell your healthcare provider if you:
- have heart problems
- have high blood pressure (hypertension)
- have convulsions (seizures)
- have thyroid problems
- have diabetes
- have low potassium levels in your blood
- are pregnant or plan to become pregnant. It is not known if VENTOLIN HFA will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if VENTOLIN HFA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you are using VENTOLIN HFA.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
VENTOLIN HFA and other medicines may affect each other and cause side effects. VENTOLIN HFA may affect the way other medicines work, and other medicines may affect the way VENTOLIN HFA works.
Especially tell your healthcare provider if you take:
- other inhaled medicines or asthma medicines
- beta-blocker medicines
- diuretics
- digoxin
- monoamine oxidase inhibitors
- tricyclic antidepressants
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use VENTOLIN HFA?
- For detailed instructions, see “ Instructions for Use” at the end of this Patient Information.
- Use VENTOLIN HFA exactly as your healthcare provider tells you to use it.
- Do not increase your dose or take extra doses of VENTOLIN HFA without first talking to your healthcare provider.
- If your child needs to use VENTOLIN HFA, watch your child closely to make sure your child uses the inhaler correctly. Your healthcare provider will show you how your child should use VENTOLIN HFA.
- Each dose of VENTOLIN HFA should last up to 4 hours to 6 hours.
- Get medical help right away if VENTOLIN HFA no longer helps your symptoms.
- Get medical help right away if your symptoms get worse or if you need to use your inhaler more often.
- While you are using VENTOLIN HFA, use other inhaled medicines and asthma medicines only as directed by your healthcare provider.
- Call your healthcare provider if your asthma symptoms like wheezing and trouble breathing become worse over a few hours or days. Your healthcare provider may need to give you another medicine to treat your symptoms.
What are the possible side effects of VENTOLIN HFA?
VENTOLIN HFA may cause serious side effects, including:
- worsening trouble breathing, coughing, and wheezing (paradoxical bronchospasm). If this happens, stop using VENTOLIN HFA and call your healthcare provider or get emergency help right away. Paradoxical bronchospasm is more likely to happen with your first use of a new canister of medicine.
- heart problems, including faster heart rate and higher blood pressure
- possible death in people with asthma who use too much VENTOLIN HFA
-
allergic reactions. Call your healthcare provider right away if you have any of the following symptoms of an allergic reaction:
- itchy skin
- swelling beneath your skin or in your throat
- rash
- worsening trouble breathing
- low potassium levels in your blood
- worsening of other medical problems in people who also use VENTOLIN HFA, including increases in blood sugar
Common side effects of VENTOLIN HFA include:
- your heart feels like it is pounding or racing (palpitations)
- chest pain
- fast heart rate
- shakiness
- nervousness
- headache
- pain
- dizziness
- sore throat
- runny nose
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VENTOLIN HFA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VENTOLIN HFA?
- Store VENTOLIN HFA at room temperature between 59°F and 77°F (15°C and 25°C) with the mouthpiece down.
- Avoid exposure to extreme heat and cold.
- Shake the VENTOLIN HFA canister well before use.
- Do not puncture the VENTOLIN HFA canister.
- Do not store the VENTOLIN HFA canister near heat or a flame. Temperatures above 120°F may cause the canister to burst.
- Do not throw the VENTOLIN HFA canister into a fire or an incinerator.
- Avoid spraying VENTOLIN HFA in your eyes.
Keep VENTOLIN HFA and all medicines out of the reach of children.
General information about the safe and effective use of VENTOLIN HFA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VENTOLIN HFA for a condition for which it was not prescribed. Do not give VENTOLIN HFA to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about VENTOLIN HFA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about VENTOLIN HFA that is written for healthcare professionals.
For more information, go to www.ventolin.com or call 1-888-825-5249.
What are the ingredients in VENTOLIN HFA?
Active ingredient: albuterol sulfate
Inactive ingredient: propellant HFA-134a
Instructions for Use
VENTOLIN ® HFA (vent′ o-lin)
(albuterol sulfate)
Inhalation Aerosol
Read this Instructions for Use before you start using VENTOLIN HFA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
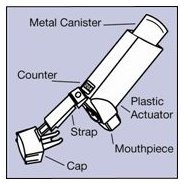
The parts of your VENTOLIN HFA inhaler:
There are 2 main parts of your VENTOLIN HFA inhaler:
- the blue plastic actuator that sprays the medicine into your mouth. See Figure A.
- the metal canister that holds the medicine. See Figure A.
Figure A
The actuator has a protective cap that covers the mouthpiece. The strap on the cap will stay attached to the actuator.
Do not use this actuator with a canister of medicine from any other inhaler.
Do not use this canister of medicine with an actuator from any other inhaler.
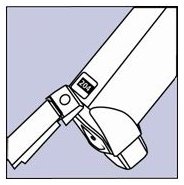
The canister has a counter that shows you how many sprays of medicine you have left. The number shows through a window in the back of the actuator. The counter starts at either 204 or 64, depending on which size inhaler you have. See Figure B.
Figure B
Priming your VENTOLIN HFA inhaler:
Your VENTOLIN HFA inhaler must be primed before you use it for the first time, when it has not been used for more than 14 days in a row, or if it has been dropped. Do not prime your VENTOLIN HFA every day.
- Remove your VENTOLIN HFA inhaler from its packaging.
- Throw away the pouch and the drying packet that comes inside the pouch.
- Remove the protective cap from the mouthpiece.
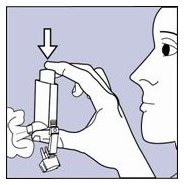
- Shake the inhaler well, and spray it into the air away from your face. See Figure C.
Figure C
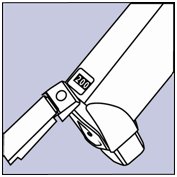
- Shake and spray the inhaler like this 3 more times to finish priming it. After you prime the actuator for the first time, the dose counter in the window on the back of the actuator should show the number 200 or 60, depending on which size inhaler you have. See Figure D.
Figure D
Each time you use your VENTOLIN HFA inhaler:
- Make sure the canister fits firmly in the plastic actuator.
- Look into the mouthpiece to make sure there are no foreign objects there, especially if the strap is no longer attached to the actuator or the cap has not been used to cover the mouthpiece.
Reading the dose counter on your VENTOLIN HFA actuator:
- The dose counter will count down by 1 number each time you spray the inhaler.
- The dose counter stops counting when it reaches 000. It will continue to show 000.
- The dose counter cannot be reset, and it is permanently attached to the metal canister. Never try to change the numbers for the dose counter or take the counter off the metal canister.
- Do not remove the canister from the plastic actuator except during cleaning to prevent accidently spraying a dose of VENTOLIN HFA into the air.
Using your VENTOLIN HFA inhaler:
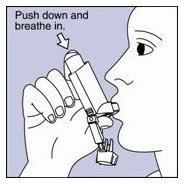
Step 1. Shake the inhaler well before each spray. Take the cap off the mouthpiece of the actuator.
- Step 2. Hold the inhaler with the mouthpiece down. See Figure E.
Figure E
- Step 3. Breathe out through your mouth and push as much air from your lungs as you can. Put the mouthpiece in your mouth and close your lips around it. See Figure F.
Figure F
- Step 4. Push the top of the canister all the way down while you breathe in deeply and slowly through your mouth. See Figure F.
- Step 5. Right after the spray comes out, take your finger off the canister. After you have breathed in all the way, take the inhaler out of your mouth and close your mouth.
Step 6. Hold your breath as long as you can, up to 10 seconds, then breathe normally.
If your healthcare provider has told you to use more sprays, wait 1 minute and shake the inhaler again. Repeat Steps 2 through Step 6.
- Step 7. Put the cap back on the mouthpiece after every time you use the inhaler. Make sure the cap snaps firmly into place.
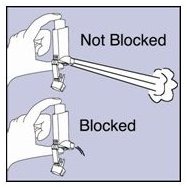
Cleaning your VENTOLIN HFA actuator:
It is very important to keep the plastic actuator clean so the medicine will not build-up and block the spray. See Figure G.
Figure G
- Do not try to clean the metal canister or let it get wet. The inhaler may stop spraying if it is not cleaned correctly.
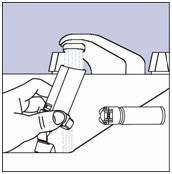
- Wash the actuator at least once a week as follows:
- Step 8. Take the canister out of the actuator, and take the cap off the mouthpiece. The strap on the cap will stay attached to the actuator.
- Step 9. Hold the actuator under the faucet and run warm water through it for about 30 seconds. See Figure H.
Figure H
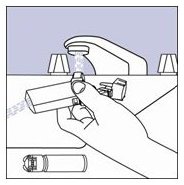
- Step 10. Turn the actuator upside down and run warm water through the mouthpiece for about 30 seconds. See Figure I.
Figure I
- Step 11. Shake off as much water from the actuator as you can. Look into the mouthpiece to make sure any medicine build-up has been completely washed away. If there is any build-up, repeat Steps 9 and 10.
- Step 12. Let the actuator air-dry completely, such as overnight. See Figure J.
Figure J
- Step 13. When the actuator is dry, put the canister in the actuator and make sure it fits firmly. Shake the inhaler well and spray it once into the air away from your face. (The counter will count down by 1 number.) Put the cap back on the mouthpiece.
If you need to use your inhaler before the actuator is completely dry:
- Shake as much water off the actuator as you can.
- Put the canister in the actuator and make sure it fits firmly.
- Shake the inhaler well and spray it once into the air away from your face.
- Take your VENTOLIN HFA dose as prescribed.
- Follow cleaning Steps 8 through 13 above.
Replacing your VENTOLIN HFA inhaler:
- When the dose counter on the actuator shows the number 020, you need to refill your prescription or ask your doctor for another prescription for VENTOLIN HFA.
- Throw the VENTOLIN HFA inhaler away as soon as the dose counter shows 000, after the expiration date on the VENTOLIN HFA packaging, or 12 months after you open the foil pouch, whichever comes first. You should not keep using the inhaler after the dose counter shows 000 even though the canister may not be completely empty. You cannot be sure you will receive the right amount of medicine.
VENTOLIN is a registered trademark of GlaxoSmithKline.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2012, GlaxoSmithKline. All rights reserved.
October 2012
VNT:8PIL
Principal Display Panel
NDC 0173-0682-20
Ventolin ® HFA
(albuterol sulfate)
Inhalation Aerosol
90 mcg per actuation
200 Metered Inhalations
R x only
FOR ORAL INHALATION ONLY
For use with Ventolin ® HFA actuator only.
Discard when the counter reads 000 or 12 months after removal from the moisture-protective foil pouch.
Pouch opened: ____ Use by: ____
Net Wt. 18 g
Contents: A microcrystalline suspension of albuterol sulfate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). Each actuation delivers 108 mcg of albuterol sulfate equivalent to 90 mcg albuterol base from the mouthpiece. See prescribing information for dosage information.
Important: Read accompanying directions carefully.
WARNING: Do not exceed the dose prescribed by your doctor.
If difficulty in breathing persists, get immediate medical help.
Contents Under Pressure: Do not puncture. Do not use or store near heat or open flame. Keep out of reach of children. Shake the inhaler well before each spray.
Store and use between 15 o and 25 oC (59 o and 77 oF). Store inhaler with mouthpiece down.
| VENTOLIN
HFA
albuterol sulfate aerosol, metered |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Clinical Solutions Wholesale (078710347) |
| Registrant - Clinical Solutions Wholesale (078710347) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Clinical Solutions Wholesale | 078710347 | relabel(58118-0037) | |