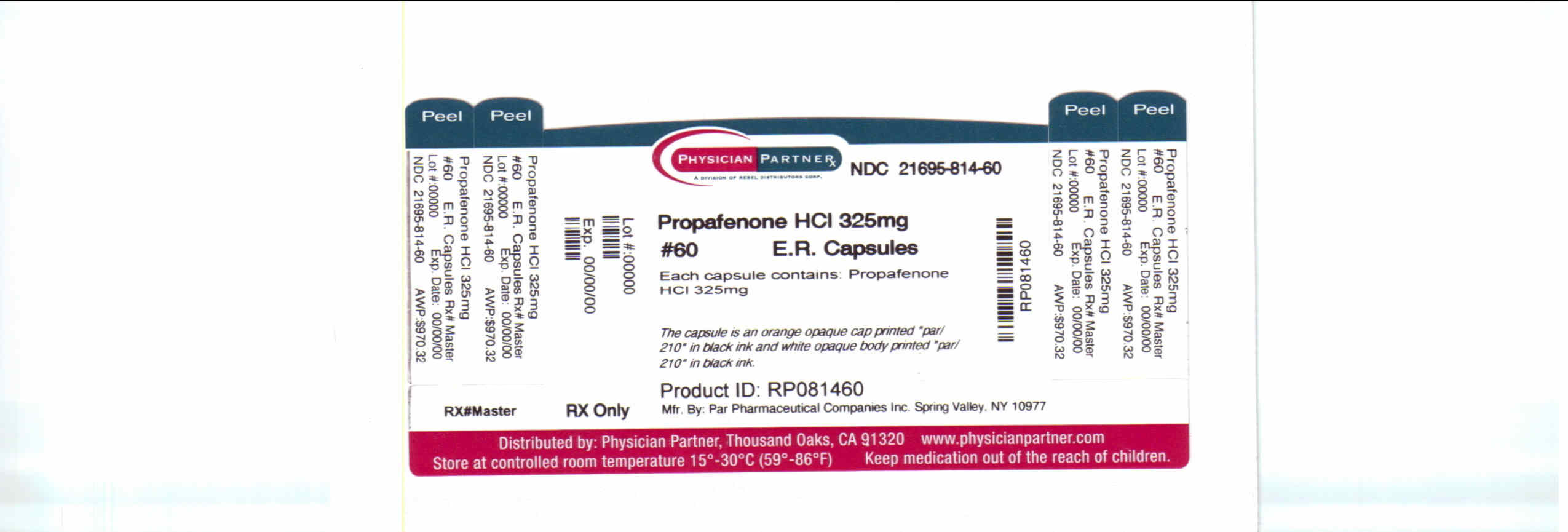
Label: PROPAFENONE- propafenone hydrochloride capsule, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-814-60 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 49884-210
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 13, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Propafenone hydrochloride is an antiarrhythmic drug supplied in extended-release capsules of 225 mg, 325 mg and 425 mg for oral administration.
The structural formula of propafenone HCl is given below:
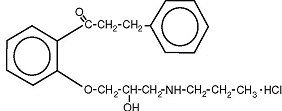
Propafenone HCl has some structural similarities to beta-blocking agents. Propafenone HCl occurs as colorless crystals or white crystalline powder with a very bitter taste. It is slightly soluble in water (20ºC), chloroform and ethanol. Propafenone extended release are capsules filled with granules containing the following inactive ingredients: ethylcellulose, lactose anhydrous, magnesium stearate and povidone. The capsules consist of D&C Red #28, FD&C Blue #1, FD&C Red #40, FD&C Yellow #5, FD&C Yellow #6, gelatin and titanium dioxide. In addition the ink consists of D&C Yellow #10 aluminum lake, iron oxide black, n-butyl alcohol, propylene glycol, FD&C Blue #2 aluminum lake, FD&C Red #40 aluminum lake, FD&C Blue #1 aluminum lake and shellac glaze~45% (20% esterfied) in ethanol.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Propafenone is a Class 1C antiarrhythmic drug with local anesthetic effects, and a direct stabilizing action on myocardial membranes. The electrophysiological effect of propafenone manifests itself in a reduction of upstroke velocity (Phase 0) of the monophasic action potential. In Purkinje fibers, and to a lesser extent myocardial fibers, propafenone reduces the fast inward current carried by sodium ions. Diastolic excitability threshold is increased and effective refractory period prolonged. Propafenone reduces spontaneous automaticity and depresses triggered activity.
Studies in anesthetized dogs and isolated organ preparations show that propafenone has beta-sympatholytic activity at about 1/50 the potency of propranolol. Clinical studies employing isoproterenol challenge and exercise testing after single doses of propafenone indicate a beta-adrenergic blocking potency (per mg) about 1/40 that of propranolol in man. In clinical trials with the immediate release formulation, resting heart rate decreases of about 8% were noted at the higher end of the therapeutic plasma concentration range. At very high concentrations in vitro, propafenone can inhibit the slow inward current carried by calcium, but this calcium antagonist effect probably does not contribute to antiarrhythmic efficacy. Moreover, propafenone inhibits a variety of cardiac potassium currents in in vitro studies (i.e. the transient outward, the delayed rectifier, and the inward rectifier current). Propafenone has local anesthetic activity approximately equal to procaine. Compared to propafenone, the main metabolite, 5-hydroxypropafenone, has similar sodium and calcium channel activity, but about 10 times less beta-blocking activity (N-depropylpropafenone has weaker sodium channel activity but equivalent affinity for beta-receptors).
Electrophysiology:
Electrophysiology studies in patients with ventricular tachycardia (VT) have shown that propafenone prolongs atrioventricular (AV) conduction while having little or no effect on sinus node function. Both atrioventricular (AV) nodal conduction time (AH interval) and His-Purkinje conduction time (HV interval) are prolonged. Propafenone has little or no effect on the atrial functional refractory period, but AV nodal functional and effective refractory periods are prolonged. In patients with Wolff-Parkinson-White (WPW) syndrome, propafenone hydrochloride immediate release tablets reduce conduction and increase the effective refractory period of the accessory pathway in both directions (see ADVERSE REACTIONS/ Electrocardiograms).
Hemodynamics:
Studies in humans have shown that propafenone exerts a negative inotropic effect on the myocardium. Cardiac catheterization studies in patients with moderately impaired ventricular function (mean C.I.=2.61 L/min/m2), utilizing intravenous propafenone infusions (loading dose of 2 mg/kg over 10 min+ followed by 2 mg/min for 30 min) that gave mean plasma concentrations of 3.0 mcg/mL (a dose that produces plasma levels of propafenone greater than does recommended oral dosing), showed significant increases in pulmonary capillary wedge pressure, systemic and pulmonary vascular resistances and depression of cardiac output and cardiac index.
Pharmacokinetics and Metabolism:
Absorption/Bioavailability
Maximal plasma levels of propafenone are reached between three to eight hours following the administration of propafenone hydrochloride extended release. Propafenone is known to undergo extensive and saturable presystemic biotransformation which results in a dose and dosage form dependent absolute bioavailability; e.g., a 150 mg immediate release tablet had an absolute bioavailability of 3.4%, while a 300 mg immediate release tablet had an absolute bioavailability of 10.6%. Absorption from a 300 mg solution dose was rapid, with an absolute bioavailability of 21.4%. At still larger doses, above those recommended, bioavailability of propafenone from immediate release tablets increased still further.
Relative bioavailability assessments have been performed between propafenone HCl ER capsules and propafenone HCl immediate release tablets. In extensive metabolizers, the bioavailability of propafenone from the SR formulation was less than that of the immediate release formulation as the more gradual release of propafenone from the prolonged-release preparations resulted in an increase in overall first pass metabolism (see Metabolism). As a result of the increased first pass effect, higher daily doses of propafenone were required from the SR formulation relative to the immediate release formulation, to obtain similar exposure to propafenone. The relative bioavailability of propafenone from the 325 twice daily regimens of propafenone ER approximates that of propafenone HCl immediate release 150 mg three times daily regimen. Mean exposure to 5-hydroxypropafenone was about 20-25% higher after SR capsule administration than after immediate-release tablet administration.
Food increased the exposure to propafenone 4-fold after single dose administration of 425 mg of propafenone HCl ER capsules. However, in the multiple dose study (425 mg dose BID), the difference between the fed and fasted state was not significant.
Distribution]
Following intravenous administration of propafenone, plasma levels decline in a bi-phasic manner consistent with a two compartment pharmacokinetic model. The average distribution half-life corresponding to the first phase was about five minutes. The volume of the central compartment was about 88 liters (1.1 L/kg) and the total volume of distribution about 252 liters.
In serum, propafenone is greater than 95% bound to proteins within the concentration range of 0.5 - 2 mcg/mL. Protein binding decreases to about 88% in patients with severe hepatic dysfunction.
Metabolism
There are two genetically determined patterns of propafenone metabolism. In over 90% of patients, the drug is rapidly and extensively metabolized with an elimination half-life from 2-10 hours. These patients metabolize propafenone into two active metabolites: 5-hydroxypropafenone which is formed by CYP2D6 and N-depropylpropafenone (norpropafenone) which is formed by both CYP3A4 and CYP1A2. In less than 10% of patients, metabolism of propafenone is slower because the 5-hydroxy metabolite is not formed or is minimally formed. In these patients, the estimated propafenone elimination half-life ranges from 10-32 hours. Decreased ability to form the 5-hydroxy metabolite of propafenone is associated with a diminished ability to metabolize debrisoquine and a variety of other drugs such as encainide, metoprolol, and dextromethorphan whose metabolism is mediated by the CYP2D6 isozyme. In these patients, the N-depropylpropafenone metabolite occurs in quantities comparable to the levels occurring in extensive metabolizers.
As a consequence of the observed differences in metabolism, administration of propafenone ER capsules to slow and extensive metabolizers results in significant differences in plasma concentrations of propafenone, with slow metabolizers achieving concentrations about twice those of the extensive metabolizers at daily doses of 850 mg/day. At low doses the differences are greater, with slow metabolizers attaining concentrations about three to four times higher than extensive metabolizers. In extensive metabolizers, saturation of the hydroxylation pathway (CYP2D6) results in greater-than-linear increases in plasma levels following administration of propafenone ER capsules. In slow metabolizers, propafenone pharmacokinetics are linear. Because the difference decreases at high doses and is mitigated by the lack of the active 5-hydroxy metabolite in the slow metabolizers, and because steady state conditions are achieved after four to five days of dosing in all patients, the recommended dosing regimen of propafenone ER capsules is the same for all patients. The large inter-subject variability in blood levels require that the dose of the drug be titrated carefully in patients with close attention paid to clinical and ECG evidence of toxicity (see DOSAGE AND ADMINISTRATION).
The 5-hydroxypropafenone and norpropafenone metabolites have electrophysiologic properties similar to propafenone in vitro. In man after administration of propafenone ER capsules, the 5-hydroxypropafenone metabolite is usually present in concentrations less than 40% of propafenone. The norpropafenone metabolite is usually present in concentrations less than 10% of propafenone.
Inter-Subject Variability
With propafenone, there is a considerable degree of inter-subject variability in pharmacokinetics which is due in large part to the first pass hepatic effect and non-linear pharmacokinetics in extensive metabolizers. A higher degree of inter-subject variability in pharmacokinetic parameters of propafenone was observed following both single and multiple dose administration of propafenone ER capsules. Inter-subject variability appears to be substantially less in the poor metabolizer group than in the extensive metabolizer group, suggesting that a large portion of the variability is intrinsic to CYP2D6 polymorphism rather than to the formulation.
The clearance of propafenone is reduced and the elimination half-life increased in patients with significant hepatic dysfunction (see PRECAUTIONS). Decreased liver function also increases the bioavailability of propafenone. Absolute bioavailability assessments have not been determined for the propafenone ER capsule formulation. Absolute bioavailability of propafenone HCl immediate release tablets has been demonstrated to be inversely related to indocyanine green clearance, reaching 60-70% at clearances of 7 mL/min and below.
Stereochemistry
Propafenone ER capsule is a racemic mixture. The R- and S-enantiomers of propafenone display stereoselective disposition characteristics. In vitro and in vivo studies have shown that the R-isomer of propafenone is cleared faster than the S-isomer via the 5-hydroxylation pathway (CYP2D6). This results in a higher ratio of S-propafenone to R-propafenone at steady state. Both enantiomers have equivalent potency to block sodium channels; however, the S-enantiomer is a more potent ß-antagonist than the R-enantiomer. Following administration of propafenone HCl immediate release tablets or propafenone ER capsules, the S/R ratio for the area under the plasma concentration-time curve was about 1.7. The S/R ratios of propafenone obtained after administration of 225, 325 and 425 mg propafenone ER capsules are independent of dose. In addition, no difference in the average values of the S/R ratios is evident between genotypes or over time.
Clinical Trials
Propafenone ER capsules has been evaluated in patients with a history of electrocardiographically documented recurrent episodes of symptomatic atrial fibrillation in two randomized, double-blind, placebo controlled trials.
RAFT]
In one U.S. multicenter study (propafenone ER capsules Atrial Fibrillation Trial, RAFT), three doses of propafenone ER capsules (225 mg BID, 325 mg BID and 425 mg BID) and placebo were compared in 523 patients with symptomatic, episodic atrial fibrillation. The patient population in this trial was 59% male with a mean age of 63 years, 91% White and 6% Black. The patients had a median history of atrial fibrillation of 13 months, and documented symptomatic atrial fibrillation within 12 months of study entry. Over 90% were NYHA Class I, and 21% had a prior electrical cardioversion. At baseline, 24% were treated with calcium channel blockers, 37% with beta blockers, and 38% with digoxin. Symptomatic arrhythmias after randomization were documented by transtelephonic electrocardiogram and centrally read and adjudicated by a blinded adverse event committee. Propafenone ER capsules administered for up to 39 weeks was shown to prolong significantly the time to the first recurrence of symptomatic atrial arrhythmia, predominantly atrial fibrillation, from Day 1 of randomization (primary efficacy variable) compared to placebo, as shown in Table 1.
Table 1: Analysis of tachycardia-free period (days) from Day 1 randomization * Fewer than 50% of the patients had events. The median time is not calculable.
† Terminating events comprised 91% atrial fibrillation, 5% atrial flutter, and 4% PSVT
Propafenone HCl ER Dose Parameter 225 mg BID (N=126) n(%) 325 mg BID (N=135) n(%) 425 mg BID (N=136) n(%) Placebo (N=126) n(%) Patients completing with terminating event† 66(52) 56(41) 41(30) 87(69) Comparison of tachycardia-free periods Kaplan-Meier Median 112 291 * 41 Range 0-285 0-293 0-300 0-289 p-Value (Log-rank test) 0.014 <0.0001 <0.0001 -- Hazard Ratio compared to Placebo 0.67 0.43 0.35 -- 95% CI for Hazard Ratio (0.49,0.93) (0.31,0.61) (0.24,0.51) -- There was a dose response for propafenone ER for the tachycardia-free period as shown in the proportional hazard analysis and the Kaplan-Meier curves presented in Figure 1.
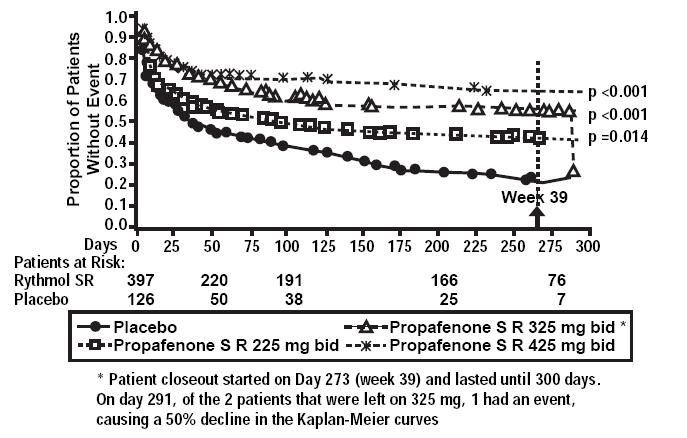
In additional analyses, propafenone ER capsules (225 mg BID, 325 mg BID, and 425 mg BID) was also shown to prolong time to the first recurrence of symptomatic atrial fibrillation from Day 5 (steady state pharmacokinetics were attained). The antiarrhythmic effect of propafenone ER capsules was not influenced by age, gender, history of cardioversion, duration of atrial fibrillation, frequency of atrial fibrillation or use of medication that lowers heart rate. Similarly, the antiarrhythmic effect of propafenone ER capsules was not influenced by the individual use of calcium channel blockers, beta-blockers or digoxin. Too few non-White patients were enrolled to assess the influence of race on effects of propafenone ER capsules (propafenone hydrochloride).
No difference in the average heart rate during the first recurrence of symptomatic arrhythmia between propafenone ER capsules and placebo was observed.
ERAFT
In a European multicenter trial [(European Rythmonorm SR Atrial Fibrillation Trial (ERAFT)], two doses of propafenone ER capsules (325 mg BID and 425 mg BID) and placebo were compared in 293 patients. The patient population in this trial was 61% male, 100% White with a mean age of 61 years. Patients had a median duration of atrial fibrillation of 3.3 years, and 61% were taking medications that lowered heart rate. At baseline, 15% of the patients were treated with calcium channel blockers (verapamil and diltiazem), 42% with beta-blockers and 8% with digoxin. During a qualifying period of up to 28 days, patients had to have one ECG-documented incident of symptomatic atrial fibrillation. The double-blind treatment phase consisted of a four day loading period followed by a 91-day efficacy period. Symptomatic arrhythmias were documented by electrocardiogram monitoring.
In ERAFT, propafenone ER capsules were shown to prolong the time to the first recurrence of symptomatic atrial arrhythmia from Day 5 of randomization (primary efficacy analysis). The proportional hazard analysis revealed that both propafenone ER capsules doses were superior to placebo. The antiarrhythmic effect of propafenone ER was not influenced by age, gender, duration of atrial fibrillation, frequency of atrial fibrillation or use of medication that lowers heart rate. It was also not influenced by the individual use of calcium channel blockers, beta-blockers or digoxin. Too few non-White patients were enrolled to assess the influence of race on the effects of propafenone ER capsules. There was a slight increase in the incidence of centrally diagnosed asymptomatic atrial fibrillation or atrial flutter in each of the two propafenone ER capsules treatment groups compared to placebo.
-
INDICATIONS AND USAGE
Propafenone Extended Release Capsules are indicated to prolong the time to recurrence of symptomatic atrial fibrillation in patients without structural heart disease.
The use of propafenone ER capsules in patients with permanent atrial fibrillation or in patients exclusively with atrial flutter or PSVT has not been evaluated. Propafenone ER capsules should not be used to control ventricular rate during atrial fibrillation.
The effect of propafenone ER capsules on mortality has not been determined (see black box WARNINGS).
-
CONTRAINDICATIONS
Propafenone ER capsules are contraindicated in the presence of congestive heart failure, cardiogenic shock, sinoatrial, atrioventricular and intraventricular disorders of impulse generation or conduction (e.g., sick sinus node syndrome, atrioventricular block) in the absence of an artificial pacemaker, bradycardia, marked hypotension, bronchospastic disorders, electrolyte imbalance, or hypersensitivity to the drug.
-
WARNINGS
Mortality:
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously, an increased rate of death or reversed cardiac arrest rate (7.7%; 56/730) was seen in patients treated with encainide or flecainide (Class 1C antiarrhythmics) compared with that seen in patients assigned to placebo (3.0%; 22/725). The average duration of treatment with encainide or flecainide in this study was ten months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) or other antiarrhythmic drugs is uncertain, but at present, it is prudent to consider any 1C antiarrhythmic to have a significant risk in patients with structural heart disease. Given the lack of any evidence that these drugs improve survival, antiarrhythmic agents should generally be avoided in patients with non-life-threatening ventricular arrhythmias, even if the patients are experiencing unpleasant, but not life-threatening, symptoms or signs.
Proarrhythmic Effects:
Propafenone has caused new or worsened arrhythmias. Such proarrhythmic effects include sudden death and life-threatening ventricular arrhythmias such as ventricular fibrillation, ventricular tachycardia, asystole and Torsade de Pointes. It may also worsen premature ventricular contractions or supraventricular arrhythmias, and it may prolong the QT interval. It is therefore essential that each patient given propafenone ER capsules be evaluated electrocardiographically prior to and during therapy, to determine whether the response to propafenone ER capsules supports continued treatment. Because propafenone prolongs the QRS interval in the electrocardiogram, changes in the QT interval are difficult to interpret.
In a 474 patient U.S. uncontrolled, open label multicenter trial using the immediate release formulation in patients with symptomatic SVT, 1.9% (9/474) of these patients experienced ventricular tachycardia (VT) or ventricular fibrillation (VF) during the study. However, in four of the nine patients, the ventricular tachycardia was of atrial origin. Six of the nine patients that developed ventricular arrhythmias did so within 14 days of onset of therapy. About 2.3% (11/474) of all patients had recurrence of SVT during the study which could have been a change in the patients’ arrhythmia behavior or could represent a proarrhythmic event. Case reports in patients treated with propafenone HCl for atrial fibrillation/flutter have included increased PVCs, VT, VF, Torsade de Pointes, asystole, and death.
In the RAFT study, there were five deaths, three in the pooled propafenone ER capsules group (0.8%) and two in the placebo group (1.6%). In the overall propafenone ER capsules and propafenone HCl immediate release database of eight studies, the mortality rate was 2.5% per year on propafenone HCl and 4% per year on placebo. Concurrent use of propafenone with other antiarrhythmic agents has not been well studied.
Use with Drugs that Prolong the QT Interval and Antiarrhythmic Agents:
The use of propafenone ER capsules (propafenone hydrochloride) in conjunction with other drugs that prolong the QT interval has not been extensively studied and is not recommended. Such drugs may include many antiarrhythmics, some phenothiazines, cisapride, bepridil, tricyclic antidepressants and oral macrolides. Class Ia and III antiarrhythmic agents should be withheld for at least five half-lives prior to dosing with propafenone ER capsules. The use of propafenone with Class Ia and III antiarrhythmic agents (including quinidine and amiodarone) is not recommended. There is only limited experience with the concomitant use of Class Ib or Ic antiarrhythmics.
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
Patients with bronchospastic disease should not, in general, receive propafenone or other agents with beta-adrenergic-blocking activity.
Congestive Heart Failure
Propafenone exerts a negative inotropic activity on the myocardium as well as beta-blockade effects and may provoke overt congestive heart failure. In the U.S. trial (RAFT) in patients with symptomatic atrial fibrillation, congestive heart failure was reported in four (1%) patients receiving propafenone ER capsules (all doses), compared to one (0.8%) patient receiving placebo. Proarrhythmic effects are more likely to occur when propafenone is administered to patients with congestive heart failure (NYHA III and IV) or severe myocardial ischemia (see CONTRAINDICATIONS).
Conduction Disturbances:
Propafenone causes dose-related first degree AV block. Average PR interval prolongation and increases in QRS duration are also dose-related.
Propafenone should not be given to patients with atrioventricular and intraventricular conduction defects in the absence of a pacemaker (see CONTRAINDICATIONS).
In a U.S. trial (RAFT) in 523 patients with a history of symptomatic atrial fibrillation treated with propafenone ER capsules, electrocardiograms obtained in response to symptoms were associated with no patients having sinus rhythm with Mobitz Type I (Wenckenbach) second degree AV block, sinus rhythm with Mobitz Type II second degree AV block, or third degree AV block. Sinus bradycardia (rate <50 beats/min) was reported with the same frequency with propafenone ER capsules and placebo.
Effects on Pacemaker Threshold:
Propafenone may alter both pacing and sensing thresholds of artificial pacemakers. Pacemakers should be monitored and programmed accordingly during therapy.
Hematologic Disturbances:
Agranulocytosis (fever, chills, weakness, and neutropenia) has been reported in patients receiving propafenone. Generally, the agranulocytosis occurred within the first two months of propafenone therapy and upon discontinuation of therapy, the white count usually normalized by 14 days. Unexplained fever and/or decrease in white cell count, particularly during the initial three months of therapy, warrant consideration of possible agranulocytosis or granulocytopenia. Patients should be instructed to report promptly the development of any signs of infection such as fever, sore throat, or chills.
-
PRECAUTIONS
Hepatic Dysfunction
Propafenone is highly metabolized by the liver and should, therefore, be administered cautiously to patients with impaired hepatic function. Severe liver dysfunction increases the bioavailability of propafenone to approximately 70% compared to 3-40% in patients with normal liver function when given propafenone HCl immediate release tablets. In eight patients with moderate to severe liver disease administered propafenone HCl immediate release tablets, the mean half-life was approximately nine hours. No studies are currently available comparing bioavailability of propafenone from propafenone ER capsules in patients with normal and impaired hepatic function. Increased bioavailability of propafenone in these patients may result in excessive accumulation. Careful monitoring for excessive pharmacological effects (see OVERDOSAGE) should be performed for patients with impaired hepatic function.
Renal Dysfunction
Approximately 50% of propafenone metabolites are excreted in the urine following administration of propafenone HCl immediate release tablets. No studies have been performed to assess the percentage of metabolites eliminated in the urine following the administration of propafenone ER capsules.
Until further data are available, propafenone ER capsules should be administered cautiously to patients with impaired renal function. These patients should be carefully monitored for signs of overdosage (see OVERDOSAGE).
Information for Patients
Medications and Supplements:
Assessment of patients’ medication history should include all over-the-counter, prescription and herbal/natural preparations with emphasis on preparations that may affect the pharmacodynamics or kinetics of propafenone ER capsules (see WARNINGS/Use with Drugs that Prolong QT interval and Antiarrhythmic Agents). Patients should be instructed to notify their health care providers of any change in over-the-counter, prescription and supplement use. If a patient is hospitalized or is prescribed new medication for any condition, the patient must inform the health care provider of ongoing propafenone ER capsules therapy. Patients should also check with their health care providers prior to taking a new over-the-counter medicine.
Electrolyte Imbalance:
If patients experience symptoms that may be associated with altered electrolyte balance, such as excessive or prolonged diarrhea, sweating, vomiting, or loss of appetite or thirst, these conditions should be immediately reported to their health care provider.
Dosing Schedule:
Patients should be instructed NOT to double the next dose if a dose is missed. The next dose should be taken at the usual time.
Elevated ANA Titers:
Positive ANA titers have been reported in patients receiving propafenone. They have been reversible upon cessations of treatment and may disappear even in the face of continued propafenone therapy. These laboratory findings were usually not associated with clinical symptoms, but there is one published case of drug-induced lupus erythematosus (positive rechallenge); it resolved completely upon discontinuation of therapy. Patients who develop an abnormal ANA test should be carefully evaluated and, if persistent or worsening elevation of ANA titers is detected, consideration should be given to discontinuing therapy.
The 225 mg capsules contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No.5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Impaired Spermatogenesis:
Reversible disorders of spermatogenesis have been demonstrated in monkeys, dogs and rabbits after high dose intravenous administration of propafenone. Evaluation of the effects of short-term propafenone HCl administration on spermatogenesis in 11 normal subjects suggested that propafenone produced a reversible, short-term drop (within normal range) in sperm count. Subsequent evaluations in 11 patients receiving propafenone HCl chronically have found no effect of propafenone on sperm count.
Neuromuscular Dysfunction
Exacerbation of myasthenia gravis has been reported during propafenone HCl immediate release tablet therapy.
Drug Interactions
Propafenone is metabolized by CYP2D6 (major pathway) and CYP1A2 and CYP3A4. Drugs that inhibit CYP2D6 (such as desipramine, paroxetine, ritonavir, sertraline), CYP1A2 (such as amiodarone), and CYP3A4 (such as ketaconazole, ritonavir, saquinavir, erythromycin, and grapefruit juice) can be expected to cause increased plasma levels of propafenone. Appropriate monitoring is recommended when propafenone ER capsules are used together with such drugs. In addition, propafenone is an inhibitor of CYP2D6. Coadministration of propafenone with drugs metabolized by CYP2D6 (such as desipramine, imipramine, haloperidol, venlafaxine) might lead to increased plasma concentrations of these drugs. The effect of propafenone on the P-Glycoprotein transporter has not been studied.
Quinidine: Small doses of quinidine completely inhibit the CYP2D6 hydroxylation metabolic pathway, making all patients, in effect, slow metabolizers (see CLINICAL PHARMACOLOGY). Concomitant administration of quinidine (50 mg TID) with 150 mg immediate release propafenone TID decreased the clearance of propafenone by 60% in EM, making them PM. Steady state plasma concentrations increased by more than 2-fold for propafenone, and decreased 50% for 5-OH-propafenone. A 100 mg dose of quinidine increased steady state concentrations of propafenone 3-fold. Concomitant use of propafenone and quinidine is not recommended.
Digoxin:Concomitant use of propafenone and digoxin increased steady state serum digoxin exposure (AUC) in patients by 60 to 270%, and decreased the clearance of digoxin by 31 to 67%. Plasma digoxin levels of patients receiving propafenone should be monitored and digoxin dosage adjusted as needed.
Lidocaine: No significant effects on the pharmacokinetics of propafenone or lidocaine have been seen following their concomitant use in patients. However, concomitant use of propafenone and lidocaine have been reported to increase the risks of central nervous system side effects of lidocaine.
Beta-Antagonists: Concomitant use of propafenone and propranolol in healthy subjects increased propranolol plasma concentrations at steady state by 113%. In 4 patients, administration of metoprolol with propafenone increased the metoprolol plasma concentrations at steady state by 100-400%. The pharmacokinetics of propafenone was not affected by the coadministration of either propranolol or metoprolol. In clinical trials using propafenone immediate release tablets, patients who were receiving beta-blockers concurrently did not experience an increased incidence of side effects.
Warfarin: The concomitant administration of propafenone and warfarin increased warfarin plasma concentrations at steady state by 39% in healthy volunteers and prolonged the prothrombin time in patients taking warfarin. Adjustment of the warfarin dose should be guided by monitoring of the prothrombin time.
Cimetidine: Concomitant administration of propafenone immediate release tablets and cimetidine in 12 healthy subjects resulted in a 20% increase in steady state plasma concentrations of propafenone.
Rifampin: Concomitant administration of rifampin and propafenone in extensive metabolizers decreased the plasma concentrations of propafenone by 67% with a corresponding decrease of 5OH-propafenone by 65%. The concentrations of norpropafenone increased by 30%. In poor metabolizers, there was a 50% decrease in propafenone plasma concentrations and increased the AUC and Cmax of norpropafenone by 74 and 20%, respectively. Urinary excretion of propafenone and its metabolites decreased significantly. Similar results were noted in elderly patients: Both the AUC and Cmax propafenone decreased by 84%, with a corresponding decrease in AUC and Cmax of 5OH-propafenone by 69 and 57%.
Fluoxetine: Concomitant administration of propafenone and fluoxetine in extensive metabolizers increased the S propafenone Cmax and AUC by 39 and 50% and the R propafenone Cmax and AUC by 71 and 50%.
Amiodarone:Concomitant administration of propafenone and amiodarone can affect conduction and repolarization and is not recommended.
Post Marketing Reports:
Orlistat may limit the fraction of propafenone available for absorption. In post marketing reports, abrupt cessation of orlistat in patients stabilized on propafenone has resulted in severe adverse events including convulsions, atrioventricular block and acute circulatory failure.
Renal and Hepatic Toxicity in Animals:
Renal changes have been observed in the rat following six months of oral administration of propafenone HCl at doses of 180 and 360 mg/kg/day (about two and four times, respectively, the maximum recommended human daily dose [MRHD] on a mg/m² basis). Both inflammatory and non-inflammatory changes in the renal tubules, with accompanying interstitial nephritis, were observed. These changes were reversible, as they were not found in rats allowed to recover for six weeks. Fatty degenerative changes of the liver were found in rats following longer durations of administration of propafenone HCl at a dose of 270 mg/kg/day (about three times the MRHD on a mg/m² basis). There were no renal or hepatic changes at 90 mg/kg/day (equivalent to the MRHD on a mg/m² basis).
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Lifetime maximally tolerated oral dose studies in mice (up to 360 mg/kg/day, about twice the maximum recommended human oral daily dose [MRHD] on a mg/m² basis) and rats (up to 270 mg/kg/day, about three times the MRHD on a mg/m² basis) provided no evidence of a carcinogenic potential for propafenone HCl.
Propafenone HCl tested negative for mutagenicity in the Ames (salmonella) test and in the in vivo mouse dominant lethal test. It tested negative for clastogenicity in the human lymphocyte chromosome aberration assay in vitro and in rat and Chinese hamster micronucleus tests, and other in vivo tests for chromosomal aberrations in rat bone marrow and Chinese hamster bone marrow and spermatogonia.
Propafenone HCl, administered intravenously to rabbits, dogs, and monkeys, has been shown to decrease spermatogenesis. These effects were reversible, were not found following oral dosing of propafenone HCl, were seen at lethal or near lethal dose levels and were not seen in rats treated either orally or intravenously (see PRECAUTIONS, Impaired Spermatogenesis). Treatment of male rabbits for 10 weeks prior to mating at an oral dose of 120 mg/kg/day (about 2.4 times the MRHD on a mg/m² basis) or an intravenous dose of 3.5 mg/kg/day (a spermatogenesis-impairing dose) did not result in evidence of impaired fertility. Nor was there evidence of impaired fertility when propafenone HCl was administered orally to male and female rats at dose levels up to 270 mg/kg/day (about 3 times the MRHD on a mg/m² basis).
Pregnancy
Teratogenic Effects: Pregnancy Category C. Propafenone HCl has been shown to be embryotoxic (decreased survival) in rabbits and rats when given in oral maternally toxic doses of 150 mg/kg/day (about three times the maximum recommended human dose [MRHD] on a mg/m² basis) and 600 mg/kg/day (about six times the MRHD on a mg/m² basis), respectively. Although maternally tolerated doses (up to 270 mg/kg/day, about three times the MRHD on a mg/m² basis) produced no evidence of embryotoxicity in rats, post-implantation loss was elevated in all rabbit treatment groups (doses as low as 15 mg/kg/day, about 1/3 the MRHD on a mg/m² basis). There are no adequate and well-controlled studies in pregnant women. Propafenone ER capsules (propafenone hydrochloride) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-Teratogenic Effects: In a study in which female rats received daily oral doses of propafenone HCl from mid-gestation through weaning of their offspring, doses as low as 90 mg/kg/day (equivalent to the MRHD on a mg/m² basis) produced increases in maternal deaths. Doses of 360 or more mg/kg/day (four or more times the MRHD on a mg/m² basis) resulted in reductions in neonatal survival, body weight gain and physiological development.
Labor and Delivery
It is not known whether the use of propafenone during labor or delivery has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for forceps delivery or other obstetrical intervention.
Nursing Mothers
Propafenone is excreted in human milk. Caution should be exercised when propafenone ER capsules are administered to a nursing mother.
Pediatric Use
The safety and effectiveness of propafenone in pediatric patients have not been established.
Geriatric Use
Of the total number of subjects in Phase III clinical studies of propafenone ER capsules (propafenone hydrochloride) 45.7 percent were 65 and over, while 15.7 percent were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals at higher doses cannot be ruled out. The effect of age on the pharmacokinetics and pharmacodynamics of propafenone has not been studied.
-
ADVERSE REACTIONS
The data described below reflect exposure to propafenone ER capsules 225 mg BID in 126 patients, to propafenone ER capsules 325 mg BID in 135 patients, to propafenone ER capsules 425 mg BID in 136 patients, and to placebo in 126 patients for up to 39 weeks in a placebo-controlled trial (RAFT) conducted in the U.S. The most commonly reported adverse events in the trial included dizziness, chest pain, palpitations, taste disturbance, dyspnea, nausea, constipation, anxiety, fatigue, upper respiratory tract infection, influenza, first degree heart block and vomiting. The frequency of discontinuation due to adverse events was highest during the first 14 days of treatment. The majority of the patients with serious adverse events who withdrew or were discontinued recovered without sequelae.
Adverse events occurring in 2% or more of the patients in any of the RAFT propafenone ER capsules treatment groups and more common with propafenone than with placebo, excluding those that are common in the population and those not plausibly related to drug therapy, are listed in Table 2.
Table 2: Most common adverse events (≥2% in any RAFT propafenone ER treatment group and more common on propafenone than on placebo) Propafenone ER Capsules MeDRA Body System/Preferred Term 225 mg BID (N = 126) n (%) 325 mg BID (N = 135) n (%) 425 mg BID (N = 136) n (%) Placebo (N=126 n (%) Mean exposure (days) 124 149 141 91 Cardiac disorders Angina pectoris 0 (0) 0 (0) 3 (2) 0 (0) Atrial flutter 3 (2) 2 (1) 0 (0) 1 (1) AV block first degree 3 (2) 3 (2) 4 (3) 0 (0) Bradycardia 4 (3) 4 (3) 6 (4) 1 (1) Cardiac failure congestive 0 (0) 1 (1) 3 (2) 1 (1) Cardiac murmur 2 (2) 3 (2) 6 (4) 0 (0) Edema 6 (5) 18 (13) 10 (7) 8 (6) Eye disorders Vision blurred 1 (1) 1 (1) 5 (4) 0 (0) Gastrointestinal disorders Constipation 10 (8) 19 (14) 16 (12) 3 (2) Diarrhea 2 (2) 3 (2) 5 (4) 3 (2) Dry mouth 1 (1) 1 (1) 5 (4) 1 (1) Flatulence 3 (2) 3 (2) 1 (1) 0 (0) Nausea 11 (9) 15 (11) 23 (17) 11 (9) Vomiting 1 (1) 0 (0) 8 (6) 3 (2) General disorder and administration site Fatigue 14 (11) 17 (13) 17 (13) 7 (6) Weakness 4 (3) 6 (4) 6 (4) 3 (2) Infections and Infestations Upper respiratory tract infection 11 (9) 16 (12) 11 (8)) 7 (6) Investigations Blood alkaline phosphatase increased 0 (0) 0 (0) 4 (3) 0 (0) Cardioactive drug level above therapeutic 1 (1) 1 (1) 3 (2) 1 (1) Hematuria 2 (2) 2 (1) 4 (3) 3 (2) Musculoskeletal, connective tissue and bone Muscle weakness 1 (1) 5 (4) 1 (1) 0 (0) Nervous system disorders Dizziness (excluding vertigo) 29 (23) 28 (21) 29 (21) 18 (14) Headache 8 (6) 12 (9) 14 (10) 11 (9) Taste disturbance 7 (6) 18 (13) 30 (22) 1 (1) Tremor 2 (2) 0 (0) 3 (2) 1 (1) Somnolence 1 (1) 1 (1) 4 (3) 0 ( 0) Psychiatric disorders Anxiety 12 (10) 17 (13) 16 (12) 13 (10) Depression 1 (1) 4 (3) 0 (0) 2 (2) Respiratory, thoracic and mediastinal disorder Dyspnea 16 (13) 23 (17) 17 (13) 9 (7) Rales 2 (2) 1 (1) 3 (2) 0 (0) Wheezing 0 (0) 0 (0) 3 (2) 0 (0) Skin & subcutaneous tissue disorders Ecchymosis 2 (2) 3 (2) 5 (4) 0 (0) No clinically important differences in incidence of adverse reactions were noted by age, or gender. Too few non-White patients were enrolled to assess adverse events according to race. Adverse events occurring in 2% or more of the patients in any of the ERAFT propafenone ER treatment groups and not listed in Table 2 include the following: bundle branch block left, bundle branch block right, conduction disorders, sinus bradycardia and hypotension.
Other adverse events reported with propafenone clinical trials not already listed in Table 2 include the following adverse events by body and preferred term.
Blood and lymphatic system disorders: anemia; lymphadenopathy; spleen disorder; thrombocytopenia; Cardiac disorders: angina unstable; arrhythmia; atrial hypertrophy; atrioventricular block; bundle branch block left; bundle branch block right; cardiac arrest; cardiac disorder; conduction disorder; coronary artery disease; extrasystoles; myocardial infarction; nodal arrhythmia; palpitations; pericarditis; sinoatrial block; sinus arrest; sinus arrhythmia; sinus bradycardia; supraventricular extrasystoles; supraventricular tachycardia; ventricular arrhythmia; ventricular extrasystoles; ventricular hypertrophy; Ear and labyrinth disorders: hearing impaired; tinnitus; vertigo; Eye disorders: eye hemorrhage; eye inflammation; eyelid ptosis; miosis; retinal disorder; visual acuity reduced; Gastrointestinal disorders: abdominal distension; abdominal pain; dry throat; duodenitis; dyspepsia; dysphagia; eructation; gastritis; gastroesophageal reflux disease; gingival bleeding; glossitis; glossodynia; gum pain; halitosis; intestinal obstruction; melena; mouth ulceration; pancreatitis; peptic ulcer; rectal bleeding; sore throat; General disorders and administration site conditions: chest pain; feeling hot; hemorrhage; malaise; pain; pyrexia; Hepato-biliary disorders: hepatomegaly; Investigations: abnormal electrocardiogram; abnormal heart sounds; abnormal liver function tests; abnormal pulse; carotid bruit; decreased blood chloride; decreased blood pressure; decreased blood sodium; decreased hemoglobin; decreased neutrophil count; decreased platelet count; decreased prothrombin level; decreased red blood cell count; decreased weight; electrocardiogram QT prolonged; glycosuria present; heart rate irregular; increased alanine aminotransferase; increased aspartate aminotransferase; increased blood bilirubin; increased blood cholesterol; increased blood creatinine; increased blood glucose; increased blood lactate dehydrogenase; increased blood pressure; increased blood prolactin; increased blood triglycerides; increased blood urea; increased blood uric acid; increased eosinophil count; increased gamma-glutamyltransferase; increased monocyte count; increased prostatic specific antigen; increased prothrombin level; increased weight; increased white blood cell count; ketonuria present; proteinuria present; Metabolism and nutrition disorders: anorexia; dehydration; diabetes mellitus; gout; hypercholesterolemia; hyperglycemia; hyperlipidemia; hypokalemia; Musculoskeletal, connective tissue and bone disorders: arthritis; bursitis; collagen-vascular disease; costochondritis; joint disorder; muscle cramps; muscle spasms; myalgia; neck pain; pain in jaw; sciatica; tendonitis; Nervous system disorders: amnesia; ataxia; balance impaired; brain damage; cerebrovascular accident; dementia; gait abnormal; hypertonia; hypothesia; insomnia; paralysis; paresthesia; peripheral neuropathy; speech disorder; syncope; tongue hypoesthesia; Psychiatric disorders: decreased libido; emotional disturbance; mental disorder; neurosis; nightmare; sleep disorder; Renal and urinary disorders: dysuria; nocturia; oliguria; pyuria; renal failure; urinary casts; urinary frequency; urinary incontinence; urinary retention; urine abnormal; Reproductive system and breast disorders: breast pain; impotence; prostatism; Respiratory, thoracic and mediastinal disorders: atelectasis; breath sounds decreased; chronic obstructive airways disease; cough; epistaxis; hemoptysis; lung disorder; pleural effusion; pulmonary congestion; rales; respiratory failure; rhinitis; throat tightness; Skin and subcutaneous tissue disorders: alopecia; dermatitis; dry skin; erythema; nail abnormality; petechiae; pruritis; sweating increased; urticaria; Vascular disorders: arterial embolism limb; deep limb venous thrombosis; flushing; hematoma; hypertension; hypertensive crisis; hypotension; labile blood pressure; pallor; peripheral coldness; peripheral vascular disease; thrombosis.
Laboratory: Electrocardiograms
Propafenone prolongs the PR and QRS intervals in patients with atrial and ventricular arrhythmias. Prolongation of the QRS interval makes it difficult to interpret the effect of propafenone on the QT interval.
Table 3: Mean Change in 12-Lead Electrocardiogram Results (RAFT) *Calculated using Bazett’s correction factor.
Propafenone ER Capsules BID dosing 225 mg 325 mg 425 mg Placebo n=126 n=135 n=136 n=126 PR (ms) 9±22 12±23 21±24 1±16 QRS (ms) 4±14 6±15 6±15 -2±12 QTc* (ms) 2±30 5±36 6±37 5±35 In RAFT, the distribution of the maximum changes in QTc compared to baseline over the study in each patient was similar in the propafenone ER capsules 225 mg BID, 325 mg BID, and 425 mg BID and placebo dose groups. Similar results were seen in the ERAFT study.
Table 4: Number of patients according to the range of maximum QTc change compared to baseline over the study in each dose group (RAFT study) Range of maximum QTc change Propafenone ER Capsules Placebo 225 mg BID 325 mg BID 425 mg BID N=119 N=129 N=123 N=100 n (%) n (%) n (%) n (%) >20% 1 (1%) 6 (5%) 3 (2%) 5 (4%) 10-20% 19 (16%) 28 (22%) 32 (26%) 24 (20%) ≤10% 99 (83%) 95 (74%) 88 (72%) 91 (76%) -
OVERDOSAGE
The symptoms of overdosage may include hypotension, somnolence, bradycardia, intra-atrial and intraventricular conduction disturbances, and rarely convulsions and high grade ventricular arrhythmias. Defibrillation as well as infusion of dopamine and isoproterenol have been effective in controlling abnormal ventricular rhythm and blood pressure. Convulsions have been alleviated with intravenous diazepam. General supportive measures such as mechanical respiratory assistance and external cardiac massage may be necessary.
The hemodialysis of propafenone in patients with an overdose is expected to be of limited value in the removal of propafenone as a result of both its high protein binding (>95%) and large volume of distribution.
-
DOSAGE AND ADMINISTRATION
The dose of propafenone ER capsules must be individually titrated on the basis of response and tolerance. Therapy should be initiated with propafenone ER capsules 225 mg given every twelve hours. Dosage may be increased at a minimum of five day interval to 325 mg given every twelve hours. If additional therapeutic effect is needed, the dose of propafenone ER capsules may be increased to 425 mg given every twelve hours.
In patients with hepatic impairment or having significant widening of the QRS complex or second or third degree AV block, dose reduction should be considered.
Propafenone ER capsules can be taken with or without food. Do not crush or further divide the contents of the capsule.
-
HOW SUPPLIED
Propafenone Extended Release Capsules, 325 mg are available as hard gelatin capsules containing 325 mg of propafenone HCl. The capsule is an orange opaque cap printed “par/210” in black ink and white opaque body printed “par/210” in black ink.
NDC 49884-210-02 Bottles of 60 capsules
NDC 49884-210-01 Bottles of 100 capsules
NDC 49884-210-05 Bottles of 500 capsules
NDC 49884-210-10 Bottles of 1000 capsules
Storage: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP.
Manufactured by:
Par Pharmaceutical Companies, Inc.
Spring Valley, NY 10977
I03/10
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- PRINCIPAL DISPLAY PANEL – 325 MG
-
INGREDIENTS AND APPEARANCE
PROPAFENONE
propafenone hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-814(NDC:49884-210) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPAFENONE (UNII: 68IQX3T69U) (PROPAFENONE - UNII:68IQX3T69U) PROPAFENONE 325 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FERRIC OXIDE BLACK (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) SHELLAC (UNII: 46N107B71O) ALCOHOL (UNII: 3K9958V90M) ETHANOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Product Characteristics Color orange (white body) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code par;210 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-814-60 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078540 01/03/2011 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK