NEUTROGENA COMPLETE ACNE THERAPY SOLUTION- salicylic acid, avobenzone, octisalate, octocrylene, oxybenzone, and benzoyl peroxide
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neutrogena® Complete Acne Therapy Solution
Warnings
For external use only
Directions
- wet face. Squeeze into hands
- apply to face and massage gently
- rinse thoroughly
- if excessive drying of peeling occurs, reduce application to every other day
Other information
Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing and limit sun exposure while using this product and for a week afterwards.
Store at room temperature.
Inactive ingredients
Water, Sodium Methyl Cocoyl Taurate, Cocamidopropyl Betaine, Sodium Cocoamphoacetate, Sodium Chloride, Glycol Distearate, Polyethylene, Glycolic Acid, Sodium Lactate, Cocamidopropyl PG-Dimonium Chloride Phosphate, Polyquaternium-11, Disodium EDTA, Fragrance, Citric Acid, Sodium Hydroxide
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
For Sunscreen Use
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Inactive ingredients
Water, Octyldodecyl Neopentanoate, Glycerin, Emulsifying Wax NF, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Phenoxyethanol, Ethylhexylglycerin, Caprylyl Glycol, Carbomer, Triethanolamine, Methylparaben, Disodium EDTA, Ethylene Brassylate, Dipropylene Glycol, Dimethyl Heptenal
Warnings
For external use only
-
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
When using this product
- avoid unnecessary sun exposure and use a sunscreen.
- avoid contact with the eyes, lips, and mouth.
- avoid contact with hair or dyed fabrics, which may be bleached by this product.
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Directions
- clean the skin thoroughly before applying this product.
- cover the entire affected area with a thin layer one to three times daily.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
Inactive ingredients
Water, Carbomer Homopolymer type B, Ethylhexylglycerin, Sodium Hydroxide, Chlorphenesin, Disodium EDTA, Laureth-4, Hydroxypropyl Methylcellulose
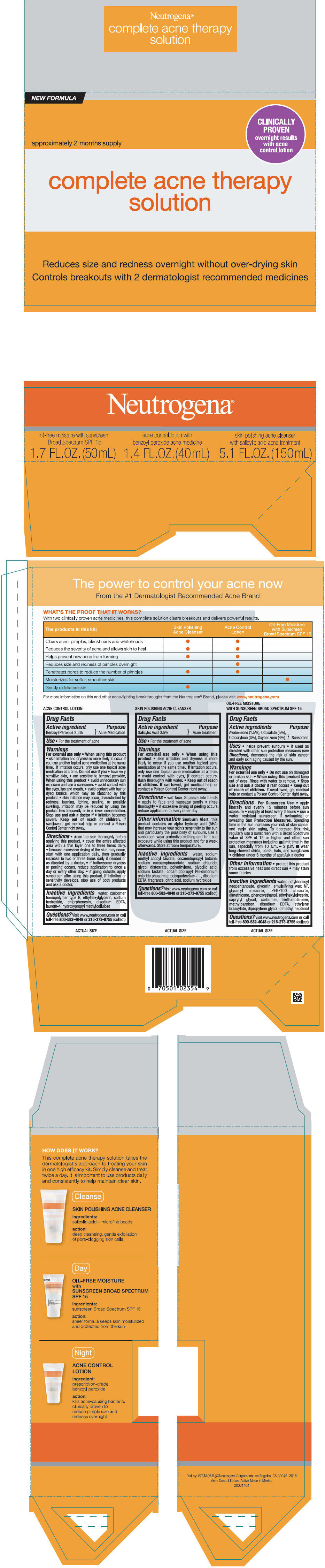
PRINCIPAL DISPLAY PANEL - Kit Carton
NEW FORMULA
approximately 2 months supply
CLINICALLY
PROVEN
overnight results
with acne
control lotion
complete acne therapy
solution
Reduces size and redness overnight without over-drying skin
Controls breakouts with 2 dermatologist recommended medicines
Neutrogena®
| oil-free moisture with sunscreen Broad Spectrum SPF 15 1.7 L. OZ. (50mL) | acne control lotion with benzoyl peroxide acne medicine 1.4 FL. OZ. (40mL) | skin polishing acne cleanser with salicylic acid acne treatment 5.1 FL.OZ. (150mL) |
| NEUTROGENA COMPLETE ACNE THERAPY SOLUTION
salicylic acid, avobenzone, octisalate, octocrylene, oxybenzone, and benzoyl peroxide kit |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |