VEMAVITE-PRX 2
WITH FISH-BASED DHA- ascorbic acid, tribasic calcium phosphate, ferrous fumarate, cholecalciferol, alpha-tocopherol, pyridoxine hydrochloride, folic acid, doconexent, and docusate sodium capsule
Trigen Laboratories, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
VemaVite™-PRx 2 Rx Prenatal Vitamin
with Fish-Based DHA
Rx Only
- 1.25 mg Folic Acid and 300 mg DHA (key omega-3 fatty acid)
- Essential vitamins and minerals
- Gentle stool softener
DESCRIPTION
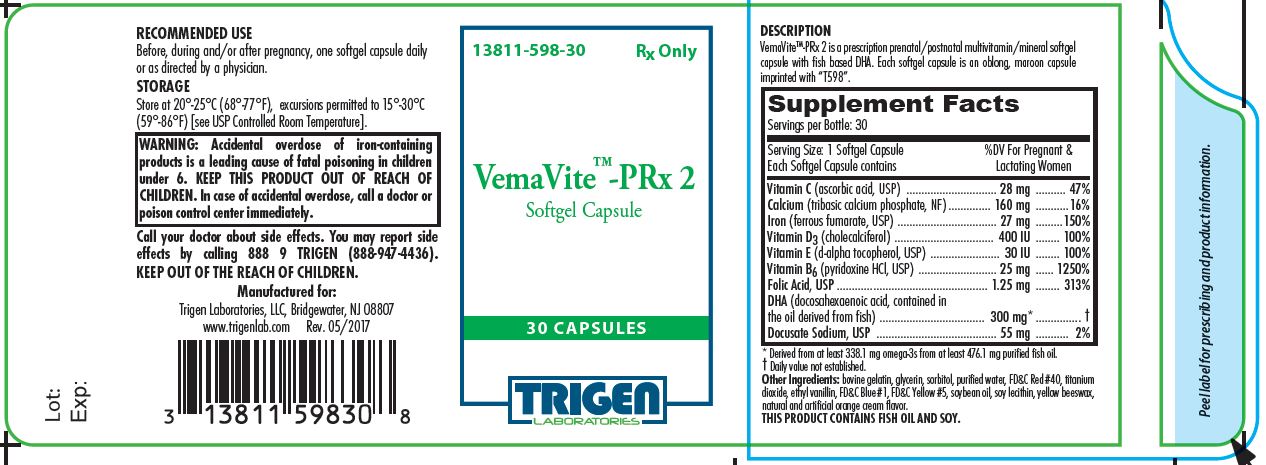
VemaVite™-PRx 2 is a prescription prenatal/postnatal multivitamin/mineral softgel capsule with fish-based DHA. Each softgel capsule is an oblong, maroon capsule imprinted with "T598".
| Each softgel capsule contains: | |
|---|---|
| *Derived from at least 338.1 mg omega-3s from at least 476.1 mg purified fish oil. | |
| Vitamin C (ascorbic acid, USP) | 28 mg |
| Calcium (tribasic calcium phosphate, NF) | 160 mg |
| Iron (ferrous fumarate, USP) | 27 mg |
| Vitamin D3 (cholecalciferol, USP) | 400 IU |
| Vitamin E (d-alpha tocopherol, USP) | 30 IU |
| Vitamin B6 (pyridoxine hydrochloride, USP) | 25 mg |
| Folic Acid, USP | 1.25 mg |
| DHA (docosahexaenoic acid, contained in the oil derived from fish) | 300 mg * |
| Docusate Sodium, USP | 55 mg |
Other Ingredients: bovine gelatin, glycerin, sorbitol, purified water, FD&C Red #40, titanium dioxide, ethyl vanillin, FD&C Blue #1, FD&C Yellow #5, soybean oil, soy lecithin, yellow beeswax, natural and artificial orange cream flavor.
INDICATIONS
VemaVite™-PRx 2 softgel capsules are indicated to provide vitamin/mineral and fish-based DHA supplementation throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. VemaVite™-PRx 2 may be useful in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS
VemaVite™-PRx 2 is contraindicated in patients with a known hypersensitivity to any of the ingredients, including fish or fish oil. Do not take this product if you are presently taking mineral oil, unless directed by a doctor.
WARNINGS
A
ccidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
THIS PRODUCT CONTAINS FISH OIL AND SOY.
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
PRECAUTION
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
CAUTION
Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) per day.
DOSAGE AND ADMINISTRATION
Before, during and/or after pregnancy, one softgel capsule daily or as directed by a physician.
HOW SUPPLIED
Bottles of 30 softgel capsules.
NDC 13811-598-30
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Store at Controlled Room Temperature 20°-25°C (68°-77°F). [See USP].
Call your doctor about side effects. You may report side effects by calling 888 9 TRIGEN (888-987-4436).
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. There are no implied or explicit claims on therapeutic equivalence.
Rx Only
Manufactured for:
TRIGEN Laboratories, Inc., Bridgewater, NJ 08807
www.trigenlab.com
Rev. 07/2017

| VEMAVITE-PRX 2
WITH FISH-BASED DHA
ascorbic acid, tribasic calcium phosphate, ferrous fumarate, cholecalciferol, alpha-tocopherol, pyridoxine hydrochloride, folic acid, doconexent, and docusate sodium capsule |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Trigen Laboratories, LLC (830479668) |