Label: PURE AND GENTLE LAXATIVE- sodium phosphate enema
- NDC Code(s): 53329-014-11, 53329-014-58
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 26, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Dosage warning: Using more than one enema in 24 hours can be harmful.
For rectal use only.
Ask a doctor before use if you
- have already used a laxative for more than 3 days
- have kidney disease, have heart problems, or are dehydrated
- are 55 years of age or older
- are on a sodium-restricted diet
- have abdominal pain, nausea or vomiting
- have a sudden change in bowel habits lasting more than 2 weeks
Ask a doctor or pharmacist before use if you
are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days, without asking a doctor
-
Directions
(or as directed by a doctor)
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
adults & children
12 years and over
1 bottle once daily
children 2 to under
12 years
Use Pediatric Enema
children under
2 years
DO NOT USE
CAUTION:Remove protective shield from enema tip before inserting.
Hold bottle upright, grasping bottle cap with fingers. Grasp protective shield with other hand and pull gently to remove.
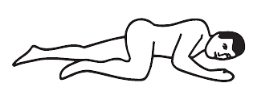
Positions for using this enema:
Left-side position: lie on left side with knee bent and arms at rest.
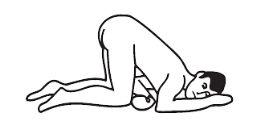
Knee-chest position: kneel, then lower head and chest forward until left side of face is resting on surface with left arm folded comfortably.
Administering enema:
- With steady pressure, gently insert enema tip into rectum with a slight side to side movement, with tip pointing toward navel. Insertion may be easier if person receiving enema bears down, as if having a bowel movement. Doing so helps relax muscles around anus.
- Do not force enema tip into rectum. Stop using if tip is hard to insert. Forcing the tip into the rectum can cause injury (especially if you have hemorrhoids). If enema tip causes rectal bleeding or pain, get immediate medical care.
- Squeeze bottle until nearly all liquid is gone. It is not necessary to empty unit completely, as it containsmore liquid than needed.
- Remove tip from rectum and stay in position until urge to evacuate is strong (usually 1 to 5 minutes). If no urge is felt after 5 minutes of using, try to empty bowel. Call a doctor promptly if no liquid comes out of the rectum after 30 minutes because dehydration could occur.
- Other Information
- Inactive ingredients
- Questions or comments?
- Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
PURE AND GENTLE LAXATIVE
sodium phosphate enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-014 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC 7 g in 118 mL SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC 19 g in 118 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-014-11 1 in 1 BOX 11/10/2017 1 133 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:53329-014-58 24 in 1 CASE 11/03/2017 2 133 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/09/2014 Labeler - Medline Industries, LP (025460908)




