THALITONE- chlorthalidone tablet
Pfizer Laboratories Div Pfizer Inc
----------
Thalitone®
(chlorthalidone tablets, USP)
15 mg
DESCRIPTION
Thalitone® (chlorthalidone USP) is an antihypertensive/diuretic supplied as 15 mg tablets for oral use. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It is a racemic mixture of 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl) benzenesulfonamide, with the following structural formula:
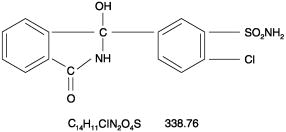
Chlorthalidone is practically insoluble in water, in ether and in chloroform; soluble in methanol; slightly soluble in alcohol.
The inactive ingredients are colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate.
CLINICAL PHARMACOLOGY
Chlorthalidone is a long-acting oral diuretic with antihypertensive activity. Its diuretic action commences a mean of 2.6 hours after dosing and continues for up to 72 hours. The drug produces diuresis with increased excretion of sodium and chloride. The diuretic effects of chlorthalidone and the benzothiadiazine (thiazide) diuretics appear to arise from similar mechanisms and the maximal effect of chlorthalidone and the thiazides appear to be similar. The site of the action appears to be the distal convoluted tubule of the nephron. The diuretic effects of chlorthalidone lead to decreased extracellular fluid volume, plasma volume, cardiac output, total exchangeable sodium, glomerular filtration rate, and renal plasma flow. Although the mechanism of action of chlorthalidone and related drugs is not wholly clear, sodium and water depletion appear to provide a basis for its antihypertensive effect. Like the thiazide diuretics, chlorthalidone produces dose-related reductions in serum potassium levels, elevations in serum uric acid and blood glucose, and it can lead to decreased sodium and chloride levels.
The mean plasma half-life of chlorthalidone is about 40 to 60 hours. It is eliminated primarily as unchanged drug in the urine. Non-renal routes of elimination have yet to be clarified. In the blood, approximately 75% of the drug is bound to plasma proteins.
Thalitone® (chlorthalidone USP) has been formulated with PVP (povidone polyvinylpyrrolidone), a bioavailability enhancer that provides 104% to 116% bioavailability relative to an oral solution of chlorthalidone. Thalitone® cannot be substituted for other formulations of chlorthalidone and likewise, other formulations of chlorthalidone cannot be substituted for Thalitone®.
INDICATIONS AND USAGE
Thalitone® (chlorthalidone USP) is indicated in the management of hypertension either alone or in combination with other antihypertensive drugs.
Chlorthalidone is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
Chlorthalidone has also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Usage in Pregnancy The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Chlorthalidone is indicated in pregnancy when edema is due to pathologic causes just as it is in the absence of pregnancy (however, see WARNINGS below). Dependent edema in pregnancy resulting from restriction of venous return by the expanded uterus is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy that is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease) but that is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort that is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
CONTRAINDICATIONS
Anuria. Known hypersensitivity to chlorthalidone or other sulfonamide-derived drugs.
WARNINGS
Thalitone® (chlorthalidone USP) should be used with caution in severe renal disease. In patients with renal disease, chlorthalidone or related drugs may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Chlorthalidone should be used with caution in patients with impaired hepatic function or progressive liver disease, because minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported with thiazide diuretics which are structurally related to chlorthalidone. However, systemic lupus erythematosus has not been reported following chlorthalidone administration.
PRECAUTIONS
General Hypokalemia and other electrolyte abnormalities, including hyponatremia and hypochloremic alkalosis, are common in patients receiving chlorthalidone. These abnormalities are dose-related but may occur even at the lowest marketed doses of chlorthalidone. Serum electrolytes should be determined before initiating therapy and at periodic intervals during therapy. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. All patients taking chlorthalidone should be observed for clinical signs of electrolyte imbalance, including dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, palpitations and gastrointestinal disturbances, such as nausea and vomiting. Digitalis therapy may exaggerate metabolic effects of hypokalemia especially with reference to myocardial activity.
Any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life-threatening. In cases of actual salt depletion, appropriate replacement is the therapy of choice.
Thiazide-like diuretics have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Calcium excretion is decreased by thiazide-like drugs. Pathological changes in the parathyroid gland with hypercalcemia and hypophosphatemia have been observed in a few patients on thiazide therapy. The common complications of hyperparathyroidism such as renal lithiasis, bone resorption and peptic ulceration have not been seen.
Uric Acid Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving chlorthalidone.
Other Increases in serum glucose may occur and latent diabetes mellitus may become manifest during chlorthalidone therapy (see PRECAUTIONS Drug Interactions). Chlorthalidone and related drugs may decrease serum PBI levels without signs of thyroid disturbance.
Information For Patients Patients should inform their doctor if they have: 1) had an allergic reaction to chlorthalidone or other diuretics or have asthma 2) kidney disease 3) liver disease 4) gout 5) systemic lupus erythematosus, or 6) been taking other drugs such as cortisone, digitalis, lithium carbonate, or drugs for diabetes.
Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle pains or cramps, nausea, vomiting or increased heart rate or pulse.
Patients should also be cautioned that taking alcohol can increase the chance of dizziness occurring.
Laboratory Tests Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
All patients receiving chlorthalidone should be observed for clinical signs of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids.
Drug Interactions Chlorthalidone may add to or potentiate the action of other antihypertensive drugs.
Insulin requirements in diabetic patients may be increased, decreased or unchanged. Higher dosage of oral hypoglycemic agents may be required.
Chlorthalidone and related drugs may increase the responsiveness to tubocurarine.
Chlorthalidone and related drugs may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
Lithium renal clearance is reduced by chlorthalidone, increasing the risk of lithium toxicity.
Drug/Laboratory Test Interactions Chlorthalidone and related drugs may decrease serum PBI levels without signs of thyroid disturbance.
Carcinogenesis, Mutagenesis, Impairment of Fertility No information is available.
Pregnancy/Teratogenic Effects PREGNANCY CATEGORY B: Reproduction studies have been performed in the rat and the rabbit at doses up to 420 times the human dose and have revealed no evidence of harm to the fetus due to chlorthalidone. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy/Non-Teratogenic Effects Thiazides cross the placental barrier and appear in cord blood. The use of chlorthalidone and related drugs in pregnant women requires that the anticipated benefits of the drug be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in the adult.
Nursing Mothers Thiazides are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from chlorthalidone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use Safety and effectiveness in children have not been established.
Geriatric Use Clinical studies of Thalitone® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
ADVERSE REACTIONS
The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency.
Gastrointestinal System Reactions: anorexia, gastric irritation, nausea, vomiting, cramping, diarrhea, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis.
Central Nervous System Reactions: dizziness, vertigo, paresthesias, headache, xanthopsia.
Hematologic Reactions: leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia.
Dermatologic-Hypersensitivity Reactions: purpura, photosensitivity, rash, urticaria, necrotizing angiitis (vasculitis)(cutaneous vasculitis), Lyell’s syndrome (toxic epidermal necrolysis).
Cardiovascular Reaction: Orthostatic hypotension may occur and may be aggravated by alcohol, barbiturates or narcotics.
Other Adverse Reactions: hyperglycemia, glycosuria, hyperuricemia, muscle spasm, weakness, restlessness, impotence.
Whenever adverse reactions are moderate or severe, chlorthalidone dosage should be reduced or therapy withdrawn.
OVERDOSAGE
Symptoms of acute overdosage include nausea, weakness, dizziness and disturbances of electrolyte balance. The oral LD50 of the drug in the mouse and the rat is more than 25,000 mg/kg body weight. The minimum lethal dose (MLD) in humans has not been established. There is no specific antidote but gastric lavage is recommended, followed by supportive treatment. Where necessary, this may include intravenous dextrose-saline with potassium, administered with caution.
DOSAGE AND ADMINISTRATION
Therapy should be initiated with the lowest possible dose, then titrated according to individual patient response. A single dose given in the morning with food is recommended; divided doses are unnecessary.
Hypertension Therapy in most patients should be initiated with a single daily dose of 15 mg. If the response is insufficient after a suitable trial, the dosage may be increased to 30 mg and then to a single daily dose of 45-50 mg. If additional control is required, the addition of a second antihypertensive drug is recommended. Increases in serum uric acid and decreases in serum potassium are dose-related over the 15-50 mg/day range and beyond.
Edema INITIATION: Adults, initially 30 to 60 mg daily or 60 mg on alternate days. Some patients may require 90 to 120 mg at these intervals or up to 120 mg daily. Dosages above this level, however, do not usually produce a greater response.
MAINTENANCE: Maintenance doses may often be lower than initial doses and should be adjusted according to the individual patient. Effectiveness is well sustained during continued use.
HOW SUPPLIED
White, kidney-shaped, compressed tablets coded M/024 containing 15 mg of chlorthalidone in bottles of 100 (NDC 61570-024-01).
Storage: Store below 30°C (86°F).
Rx Only
Prescribing Information as of January 2004.
Distributed by:
Monarch Pharmaceuticals, Inc., Bristol, TN 37620
(A wholly owned subsidiary of King Pharmaceuticals, Inc.)
Manufactured by:
King Pharmaceuticals, Inc., Bristol, TN 37620
| THALITONE
chlorthalidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| King Pharmaceuticals LLC | 962691478 | MANUFACTURE(61570-024) | |