NOTUSS PE- codeine phosphate, phenylephrine hydrochloride liquid
SJ Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NOTUSS PE
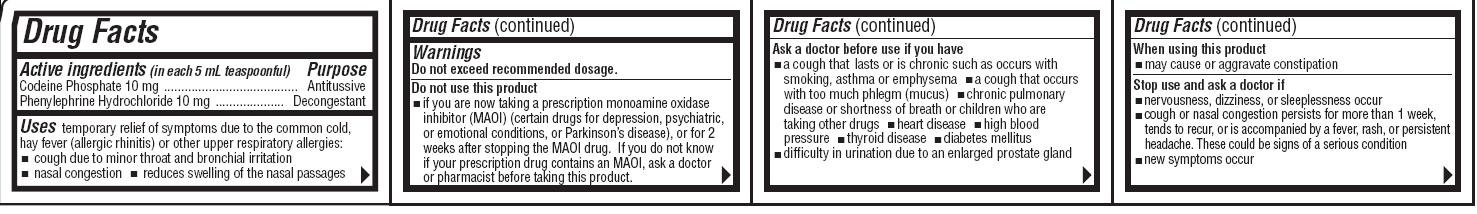
Drug Facts
Active ingredientsPurpose
(in each 5 mL
teaspoonful)
Codeine Phosphate 10 mg ............................. Antitussive
Phenylephrine Hydrochloride 10 mg ................Decongestant
Uses
temporary relief of symptoms due to the common cold, hay
fever (allergic rhinitis) or other upper respiratory allergies:
•cough due to minor throat and bronchial irritation• nasal congestion• reduces swelling of the nasal passages
Uses
temporary relief of symptoms due to the common cold, hay
fever (allergic rhinitis) or other upper respiratory allergies:
•cough due to minor throat and bronchial irritation• nasal congestion• reduces swelling of the nasal passages
Do not use this product
•if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug.If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
•a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema•a cough that occurs with too much phlegm (mucus)•chronic pulmonary disease or shortness of breath or children who are taking other drugs•heart disease•high blood pressure•thyroid disease•diabetes mellitus•difficulty in urination due to an enlarged prostate gland
Stop use and ask a doctor if
•nervousness, dizziness, or sleeplessness occur•cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. These could be signs of a serious condition. •new symptoms occur
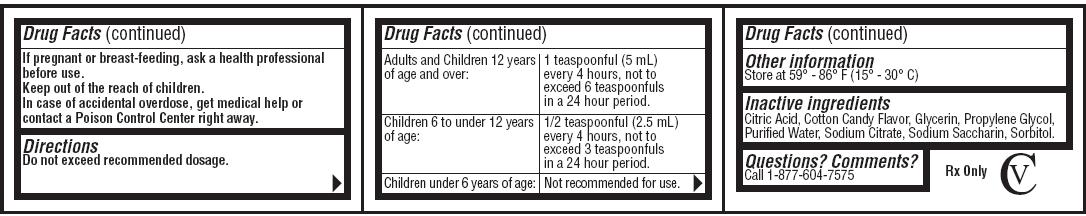
Keep out of the reach of children
In case of an accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Do not exceed recommended dosage.| Adults and children 12 years of age and over: | 1 teaspoonful (5 mL) every 4 hours, not to exceed 6 teaspoonfuls in a 24 hour period. |
| Children 6 to under 12 years of age: | 1/2 teaspoonful (2.5 mL) every 4 hours, not to exceed 3 teaspoonfuls in a 24 hour period. |
| Children under 6 years of age: | Not recommended for use. |
Inactive ingredients
Citric Acid, Cotton Candy Flavor, Glycerin,
Propylene
Glycol, Purified Water, Sodium
Citrate, Sodium Saccharin, Sorbitol.
PRODUCT PACKAGING
The packaging below represents the labeling currently used.
NDC 24839-343-16 and NDC 24839-343-10
NOTUSS-PE
Antitussive - Decongestant
Each 5 mL (one teaspoonful) for oral administration contains:
Codeine Phosphate............ 10 mg
(WARNING: May be habit forming)
Phenylephrine HCl.............. 10 mg
Rx Only
Sugar Free / Alcohol Free
Dye Free / Gluten Free
Cotton Candy Flavor
16 fl. oz. (473 mL)
SJ Pharmaceuticals
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This bottle not to be dispensed to consumer.
Manufactured by:Great Southern Laboratories
Houston, TX 77099
Distributed for:SJ Pharmaceuticals
4200 Northside Parkway NW, Bldg. 12
Atlanta, GA 30327
Iss. 06/09
Notuss PE Packaging





| NOTUSS
PE
codeine phosphate, phenylephrine hydrochloride liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - SJ Pharmaceuticals, LLC (845662720) |
| Registrant - Great Southern Laboratories (056139553) |