PREPLUS- vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, ferrous fumarate, zinc oxide, and cupric oxide tablet
Virtus Pharmaceuticals
----------
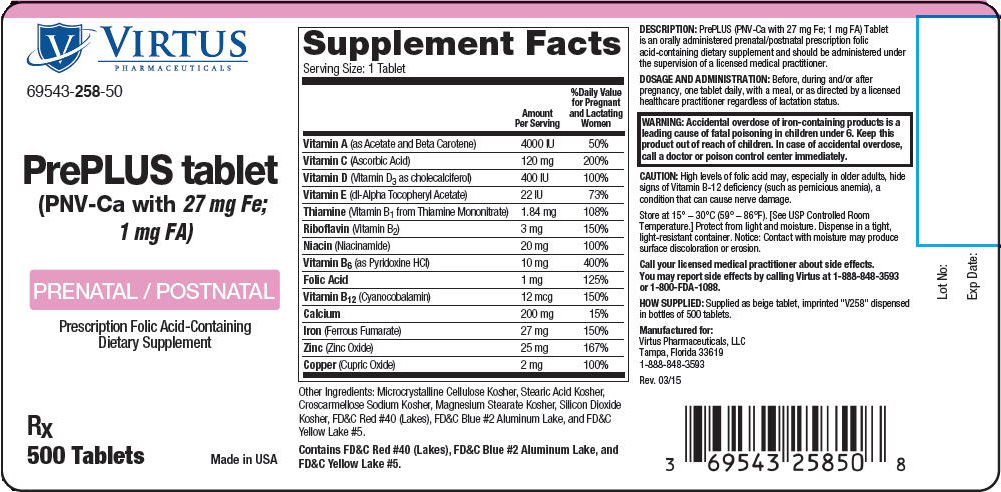
PrePLUS tablet
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | ||
| Amount Per Serving | %Daily Value for Pregnant and Lactating Women |
|
| Vitamin A (as Acetate and Beta Carotene) | 4000 IU | 50% |
| Vitamin C (Ascorbic Acid) | 120 mg | 200% |
| Vitamin D (Vitamin D3 as cholecalciferol) | 400 IU | 100% |
| Vitamin E (dl-Alpha Tocopheryl Acetate) | 22 IU | 73% |
| Thiamine (Vitamin B1 from Thiamine Mononitrate) | 1.84 mg | 108% |
| Riboflavin (Vitamin B2) | 3 mg | 150% |
| Niacin (Niacinamide) | 20 mg | 100% |
| Vitamin B6 (as Pyridoxine HCl) | 10 mg | 400% |
| Folic Acid | 1 mg | 125% |
| Vitamin B12 (Cyanocobalamin) | 12 mcg | 150% |
| Calcium | 200 mg | 15% |
| Iron (Ferrous Fumarate) | 27 mg | 150% |
| Zinc (Zinc Oxide) | 25 mg | 167% |
| Copper (Cupric Oxide) | 2 mg | 100% |
Other Ingredients: Microcrystalline Cellulose Kosher, Stearic Acid Kosher, Croscarmellose Sodium Kosher, Magnesium Stearate Kosher, Silicon Dioxide Kosher, FD&C Red #40 (Lakes), FD&C Blue #2 Aluminum Lake, and FD&C Yellow Lake #5.
Contains FD&C Red #40 (Lakes), FD&C Blue #2 Aluminum Lake, and FD&C Yellow Lake #5.
DESCRIPTION
PrePLUS (PNV-Ca with 27 mg Fe; 1 mg FA) Tablet is an orally administered prenatal/postnatal prescription folic acid-containing dietary supplement and should be administered under the supervision of a licensed medical practitioner.
DOSAGE AND ADMINISTRATION
Before, during and/or after pregnancy, one tablet daily, with a meal, or as directed by a licensed healthcare practitioner regardless of lactation status.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. |
CAUTION
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Store at 15° – 30°C (59° – 86°F). [See USP Controlled Room Temperature.] Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
| PREPLUS
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, ferrous fumarate, zinc oxide, and cupric oxide tablet |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 19 mm | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals (079659493) |