Label: TRAMAPAP- tramadol hydrochloride, acetaminophen kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 64038-059-03, 64038-135-75, 64038-738-15 - Packager: Living Well Pharmacy, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 25, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
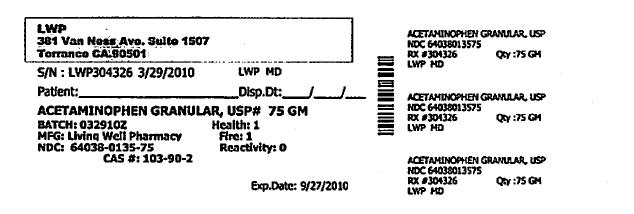
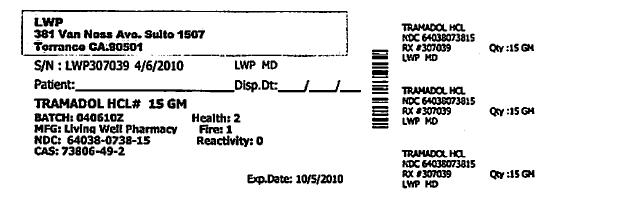
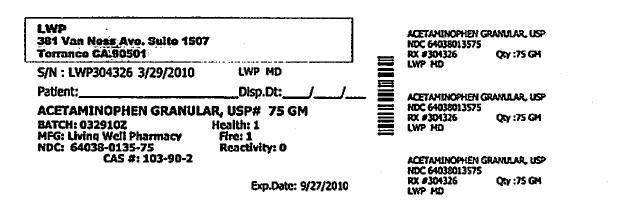
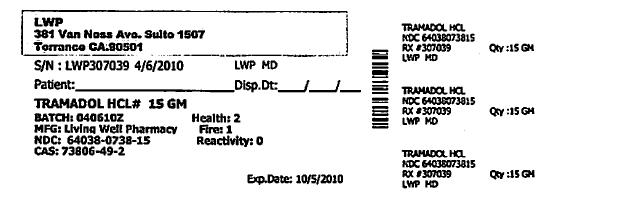
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
DESCRIPTION
- RX Only
For Prescription Compounding Only
Tramapap Kit
Tramadol HCl and Acetaminophen Compound Kit
Description
Each Tramapap Kit is comprised of 15 grams of tramadol hydrochloride powder and 75 grams of acetaminophen powder.
Certificate of Analysis on File
Tramapap Kit also contains 23.25 grams of Lactose Monohydrate (Spray dried) powder and 1.5 grams of
riboflavin USP powder. When compounded, the final product provides a homogeneous product of 300
capsules each capsule containing 50mg of tramadol and 250mg of acetaminophen.
-
INDICATIONS & USAGE
Equipment
Required supplies needed to compound this kit
Equipment Item
Quantity
Tramadol HCl (Included)
15 grams
Acetaminophen (Included)
75 grams
Lactose Monohydrate Spray Dried Powder ( Included)
23.25 grams
Riboflavin Powder (Included)
1.5 grams
Red/Blue #1 Capsules ( Required Not Included)
300 Capsules
- DRUG & OR LABORATORY TEST INTERACTIONS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- WARNINGS AND PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRAMAPAP
tramadol hydrochloride, acetaminophen kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64038-059 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64038-059-03 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 15 g Part 2 1 BOTTLE 75 g Part 1 of 2 TRAMADOL HCL
tramadol hydrochloride powderProduct Information Item Code (Source) NDC:64038-738 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tramadol Hydrochloride (UNII: 9N7R477WCK) (Tramadol - UNII:39J1LGJ30J) Tramadol Hydrochloride 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64038-738-15 15 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/07/2010 Part 2 of 2 ACETAMINOPHEN CRYSTAL
acetaminophen powderProduct Information Item Code (Source) NDC:64038-135 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64038-135-75 75 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/07/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/07/2010 Labeler - Living Well Pharmacy, Inc. (070488957) Registrant - Living Well Pharmacy, Inc. (070488957) Establishment Name Address ID/FEI Business Operations Living Well Pharmacy, Inc. 070488957 api manufacture Establishment Name Address ID/FEI Business Operations Letco 830193582 api manufacture Establishment Name Address ID/FEI Business Operations Spectrum Laboratory Products, Inc. 075295246 api manufacture Establishment Name Address ID/FEI Business Operations Medisca 627218576 api manufacture