Label: FLAVOXATE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-6326-0, 54868-6326-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 51224-154
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 25, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Flavoxate hydrochloride tablets contain flavoxate hydrochloride, a synthetic urinary tract spasmolytic.
Chemically, flavoxate hydrochloride is 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate hydrochloride. The empirical formula of flavoxate hydrochloride is C24H25NO4•HCl. The molecular weight is 427.94. The structural formula appears below.
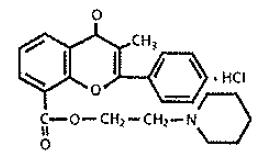
Each tablet for oral administration contains 100 mg flavoxate hydrochloride. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, dibasic calcium phosphate dihydrate, magnesium stearate, hypromellose, polydextrose. titanium dioxide and triacetin.
-
CLINICAL PHARMACOLOGY
Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle.
In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate hydrochloride was excreted in the urine within 24 hours.
-
INDICATIONS AND USAGE
Flavoxate hydrochloride tablets are indicated for symptomatic relief of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis. Flavoxate hydrochloride tablets are not indicated for definitive treatment, but are compatible with drugs used for the treatment of urinary tract infections.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Information for Patients
Patients should be informed that if drowsiness and blurred vision occur, they should not operate a motor vehicle or machinery or participate in activities where alertness is required.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of flavoxate hydrochloride have not been performed.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats and rabbits at doses up to 34 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to flavoxate hydrochloride. There are, however, no well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal: Nausea, vomiting, dry mouth.
CNS: Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic: Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular: Tachycardia and palpitation.
Allergic: Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic: Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal: Dysuria.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mg Tablets Bottle Label
-
INGREDIENTS AND APPEARANCE
FLAVOXATE HYDROCHLORIDE
flavoxate hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-6326(NDC:51224-154) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Flavoxate hydrochloride (UNII: 9C05J6089W) (Flavoxate - UNII:3E74Y80MEY) Flavoxate hydrochloride 100 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) starch, corn (UNII: O8232NY3SJ) dibasic calcium phosphate dihydrate (UNII: O7TSZ97GEP) magnesium stearate (UNII: 70097M6I30) hypromelloses (UNII: 3NXW29V3WO) polydextrose (UNII: VH2XOU12IE) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code 58 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-6326-0 90 in 1 BOTTLE 2 NDC:54868-6326-1 10 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076835 01/04/2012 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack


