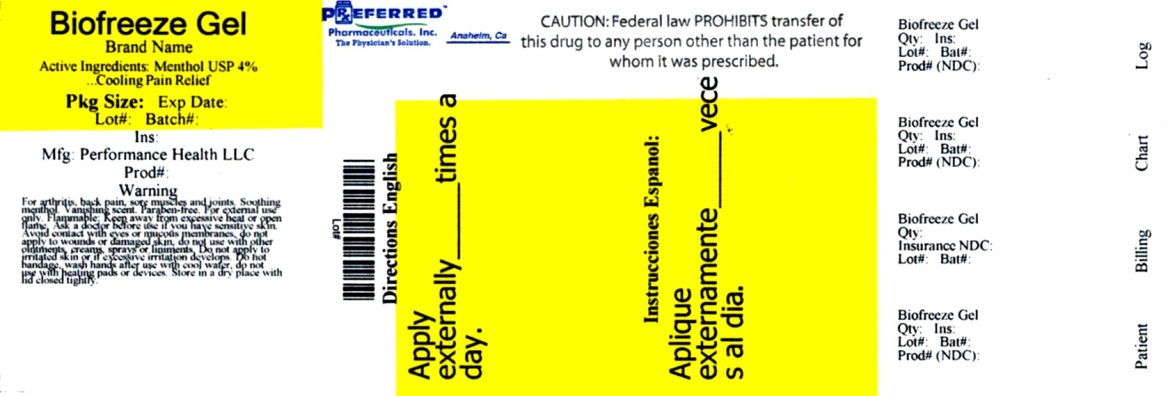
BIOFREEZE- menthol gel
Preferred Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BIOFREEZE
Uses
Temporary relief from minor aches and pains of sore muscles and joints associated with: - arthritis - backache - strains - sprains
When using this product:
- •
- Avoid contact with the eyes or mucous membranes
- •
- Do not apply to wounds or damaged skin
- •
- Do not use with other ointments, creams, sprays or liniments
- •
- Do not apply to irritated skin or if excessive irritated develops
- •
- Do not bandage
- •
- Wash hands after use with cool water
- •
- Do not use with heating pad or device
Stop use and ask a doctor if:
Condition worsens, or if symptoms persist for more than 7 days, or clear up and recur
Keep out of reach of children:
If accidentally ingested, get medical help or contact a Poison Control Center immediately
Directions:
- •
- Adults and children 2 years of age and older: Rub a thin film over affected areas not more than 4 times daily; massage not necessary
- •
-
Children under 2 years of age: Consult physician
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Arnica Montana Flower Extract, Arctium Lappa Root (Burdock) Extract, Boswellia Carterii Resin Extract, Calendula Officinalis Extract, Carbomer, Camellia Sinensis Leaf Extract , Camphor USP, Glycerin, Ilex Paraguariensis Leaf Extract (Green Tea), Isopropyl Alcohol, Isopropyl Myristate, Melissa Officinalis (Lemon Balm) Leaf Extract, Silicon Dioxide, Tocopheryl (Vitamine E) Acetate, Triethanolamine, Purified Water USP, Blue 1, Yellow 5
| BIOFREEZE
menthol gel |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-6457) | |