SELSUN BLUE FULL AND THICK- pyrithione zinc shampoo
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Selsun Blue Full and Thick
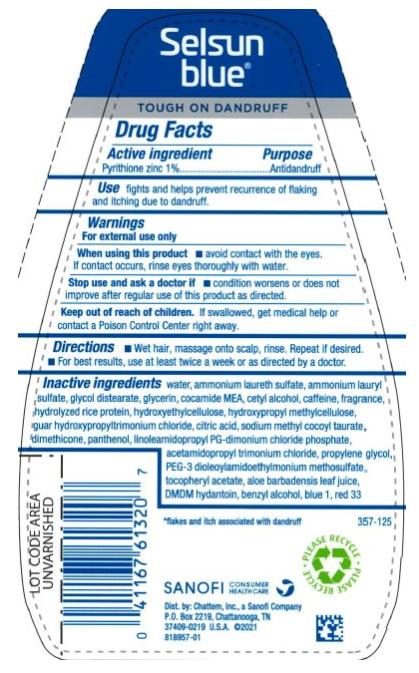
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- wet hair, massage onto scalp, rinse. Repeat if desired.
- for best results, use at least twice a week or as directed by a doctor
Inactive ingredients
water, ammonium laureth sulfate, ammonium lauryl sulfate, glycol distearate, glycerin, cocamide MEA, cetyl alcohol, fragrance, aloe barbadensis leaf juice, dimethicone, tocopheryl acetate, panthenol, acetamidopropyl trimonium chloride, linoleamidopropyl PG-dimonium chloride phosphate, PEG-3 dioleoylamidoethylmonium methosulfate, dimethiconol, sodium lauryl glucose carboxylate, lauryl glucoside, amodimethicone, hydroxypropyl methylcellulose, sodium dodecylbenzenesulfonate, laureth-9, DMDM hydantoin, benzyl alcohol, C11-15 pareth-7, trideceth-12, propylene glycol, sodium chloride, citric acid, hydroxyethylcellulose, blue 1
| SELSUN BLUE FULL AND THICK
pyrithione zinc shampoo |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |