Label: MURICIN- mupirocin ointment
- NDC Code(s): 17033-420-15
- Packager: Dechra Veterinary Products
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
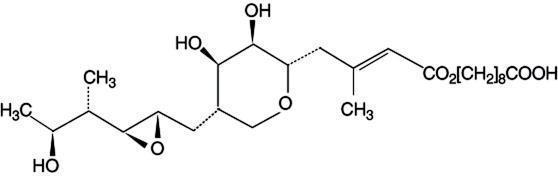
Each gram of Muricin ointment contains 20 mg of mupirocin in a bland, water-washable ointment base consisting of polyethylene glycol 400 and polyethylene glycol 3350 (polyethylene glycol ointment, NF). Mupirocin is a naturally-occurring, broadspectrum antibiotic. The chemical name is (E)-(2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The chemical structure is:
-
CLINICAL PHARMACOLOGY:
Mupirocin is a chemical entity produced by fermentation of the organism Pseudomonas fluorescens. Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this mode of action, mupirocin shows no cross resistance with chloramphenicol, erythromycin, gentamicin, lincomycin, neomycin, novobiocin, penicillin, streptomycin, and tetracycline. Mupirocin is an antimicrobial agent that inhibits the growth of Gram-positive and Gram-negative bacteria. Bacteria susceptible to the action of mupirocin in vitro include the aerobic isolates of Staphylococcus aureus (including methicillin-resistant strains and β-lactamase-producing strains), Staphylococcus intermedius, Staphylococcus epidermidis, other coagulase positive or negative Staphylococci, α-hemolytic Streptococci, β group A Streptococci (including S. pyogenes), other β Streptococci (including S. agalactiae), group D Streptococci (including S. faecalis and S. faecium), group Viridans Streptococci, Streptococcus pneumoniae, Corynebacterium hofmanii, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, Hemophilus influenzae (including β-lactamase-producing strains), Neisseria gonorrheae (including β-lactamase-producing strains), Neisseria meningitidis, Branhamella catarrhalis and Pasteurella multocida, and the anaerobic isolates of Peptostreptococcus anaerobius, Clostridium difficile, and Clostridium sporogenes.
Clinical significance of the in vitro data is unknown except for susceptible strains of Staphylococcus aureus and Staphylococcus intermedius.
- INDICATIONS FOR USE:
- CONTRAINDICATIONS:
-
WARNINGS:
- Because of the potential hazard of nephrotoxicity due to the polyethylene glycol content of the base, care should be exercised when using this product in treating extensive deep lesions where absorption of large quantities of polyethylene glycol is possible.
- Safety of use in pregnant or breeding animals has not been determined. Muricin ointment is not for ophthalmic use.
-
ADVERSE REACTIONS:
No adverse reactions have been reported with this product. If a skin reaction such as irritation should occur, treatment should be discontinued and appropriate therapy instituted.
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet, contact Dechra Veterinary Products at (866) 933-2472.
- For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
- DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
-
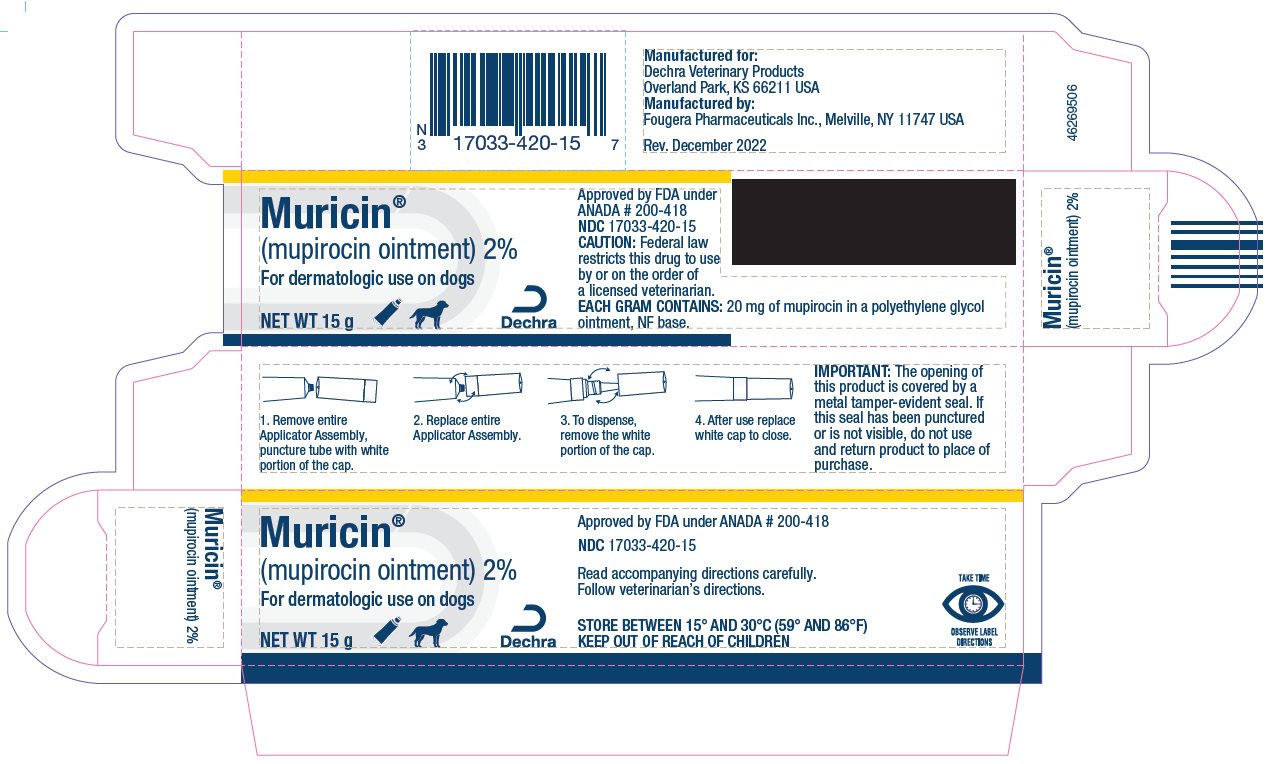
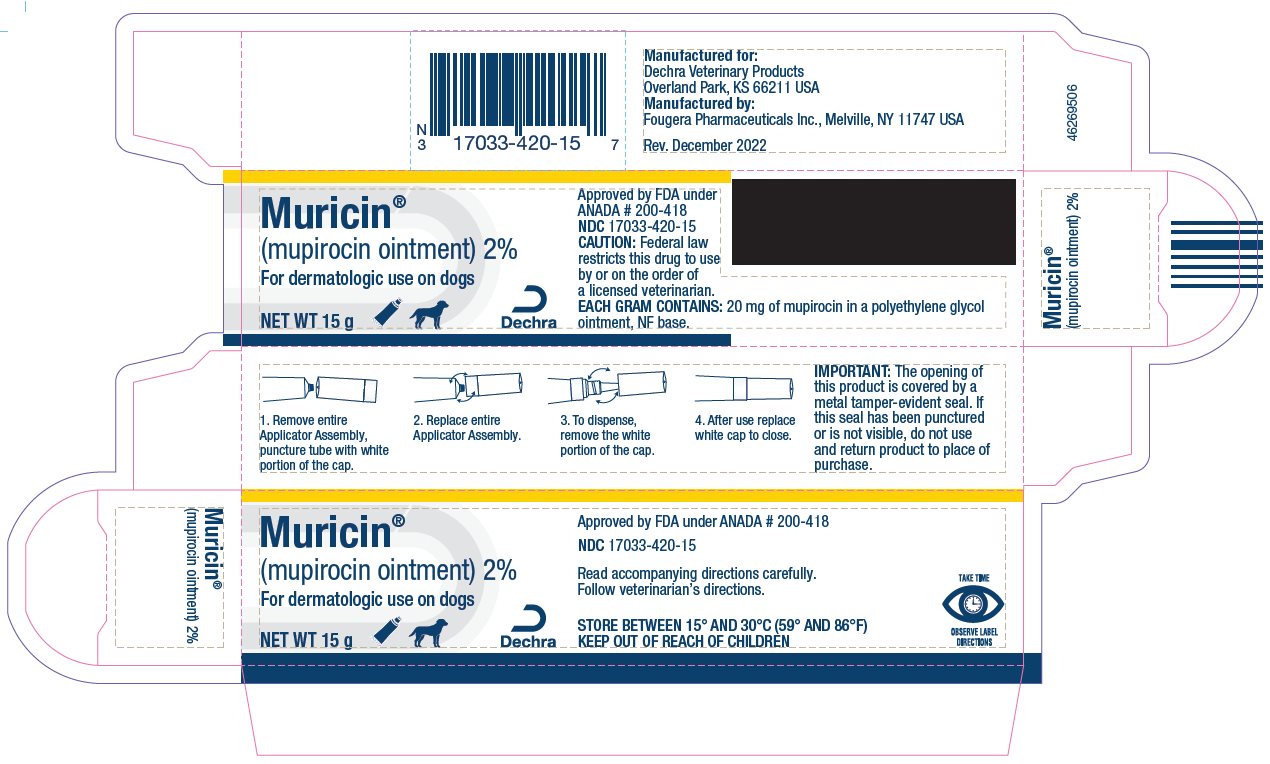
IMPORTANT:
The opening of this product is covered by a metal tamper-evident seal.
If this seal has been punctured or is not visible, do not use and return product to place of purchase.
Approved by FDA under ANADA # 200-418
Manufactured for:
Dechra Veterinary Products
Overland Park, KS 66211 USA
Manufactured by:
Fougera Pharmaceuticals Inc.
Melville, NY 11747 USA
46269504
Rev. December 2022
- PRINCIPAL DISPLAY PANEL - 15 g Carton
-
INGREDIENTS AND APPEARANCE
MURICIN
mupirocin ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MUPIROCIN (UNII: D0GX863OA5) (MUPIROCIN - UNII:D0GX863OA5) MUPIROCIN 20 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-420-15 1 in 1 CARTON 1 15 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200418 03/08/2007 Labeler - Dechra Veterinary Products (362142734) Registrant - Fougera Pharmaceuticals Inc. (043838424)